Regional Topological Aberrances of White Matter- and Gray Matter-Based Functional Networks for Attention Processing May Foster Traumatic Brain Injury-Related Attention Deficits in Adults
Abstract
:1. Introduction
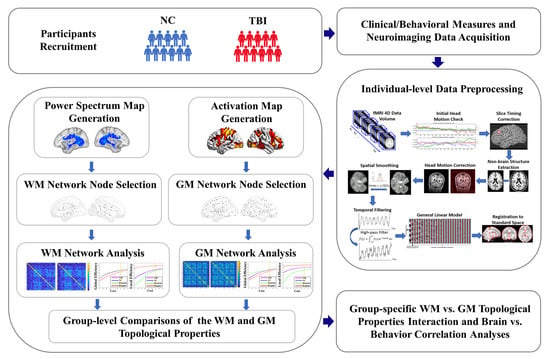
2. Materials and Methods
2.1. Participants
2.2. Experimental Task for fMRI Acquisition
2.3. Experimental Setup and MRI Data Acquisition
2.4. Individual-Level fMRI Data Preprocessing
2.5. WM Functional Network Node Selection
2.6. WM Functional Network Construction and Topological Property Estimations
2.7. GM Functional Network Node Selection
2.8. GM Functional Network Construction and Topological Properties Calculation
2.9. Group-Level Analyses
2.9.1. Demographic, Clinical/Behavioral, and Task-Performance Measures
2.9.2. Topological Properties of WM and GM Functional Networks
2.9.3. WM and GM Functional Network Interaction Analysis
2.9.4. Brain–Behavior Relationship Analysis
3. Results
3.1. Demographic, Clinical/Behavioral, and Task-Performance Measures
3.2. Topological Properties of WM and GM Networks
3.3. Interactions of WM vs. GM Network Topological Properties
3.4. Brain–Behavior Relationships
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Frieden, T.R.; Houry, D.; Baldwin, G. Report to Congress on Traumatic Brain Injury in the United States: Epidemiology and Rehabilitation; Centers for Disease Control and Prevention, National Center for Injury Prevention and Control, Division of Unintentional Injury Prevention: Atalanta, GA, USA, 2015. Available online: https://www.cdc.gov/traumaticbraininjury/pubs/congress_epi_rehab.html (accessed on 5 November 2021).
- Castellanos, N.P.; Leyva, I.; Buldu, J.M.; Bajo, R.; Paul, N.; Cuesta, P.; Ordonez, V.E.; Pascua, C.L.; Boccaletti, S.; Maestu, F.; et al. Principles of recovery from traumatic brain injury: Reorganization of functional networks. Neuroimage 2011, 55, 1189–1199. [Google Scholar] [CrossRef] [PubMed]
- Sharp, D.J.; Scott, G.; Leech, R. Network dysfunction after traumatic brain injury. Nat. Rev. Neurol. 2014, 10, 156–166. [Google Scholar] [CrossRef] [PubMed]
- Kinnunen, K.M.; Greenwood, R.; Powell, J.H.; Leech, R.; Hawkins, P.C.; Bonnelle, V.; Patel, M.C.; Counsell, S.J.; Sharp, D.J. White matter damage and cognitive impairment after traumatic brain injury. Brain 2011, 134, 449–463. [Google Scholar] [CrossRef]
- Owens, J.A.; Spitz, G.; Ponsford, J.L.; Dymowski, A.R.; Willmott, C. An investigation of white matter integrity and attention deficits following traumatic brain injury. Brain Inj. 2018, 32, 776–783. [Google Scholar] [CrossRef]
- Bramlett, H.M.; Dietrich, W.D. Long-Term Consequences of Traumatic Brain Injury: Current Status of Potential Mechanisms of Injury and Neurological Outcomes. J. Neurotrauma 2015, 32, 1834–1848. [Google Scholar] [CrossRef] [PubMed]
- Daneshvar, D.H.; Riley, D.O.; Nowinski, C.J.; McKee, A.C.; Stern, R.A.; Cantu, R.C. Long-term consequences: Effects on normal development profile after concussion. Phys. Med. Rehabil. Clin. N. Am. 2011, 22, 683–700. [Google Scholar] [CrossRef] [Green Version]
- Ruttan, L.; Martin, K.; Liu, A.; Colella, B.; Green, R.E. Long-term cognitive outcome in moderate to severe traumatic brain injury: A meta-analysis examining timed and untimed tests at 1 and 4.5 or more years after injury. Arch. Phys. Med. Rehabil. 2008, 89, S69–S76. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, R.C.; Mierzwa, A.J.; Marion, C.M.; Sullivan, G.M. White matter involvement after TBI: Clues to axon and myelin repair capacity. Exp. Neurol. 2016, 275 Pt 3, 328–333. [Google Scholar] [CrossRef] [Green Version]
- Braun, M.; Vaibhav, K.; Saad, N.M.; Fatima, S.; Vender, J.R.; Baban, B.; Hoda, M.N.; Dhandapani, K.M. White matter damage after traumatic brain injury: A role for damage associated molecular patterns. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 2614–2626. [Google Scholar] [CrossRef]
- Kumar, R.; Saksena, S.; Husain, M.; Srivastava, A.; Rathore, R.K.; Agarwal, S.; Gupta, R.K. Serial changes in diffusion tensor imaging metrics of corpus callosum in moderate traumatic brain injury patients and their correlation with neuropsychometric tests: A 2-year follow-up study. J. Head Trauma Rehabil. 2010, 25, 31–42. [Google Scholar] [CrossRef]
- Maruta, J.; Suh, M.; Niogi, S.N.; Mukherjee, P.; Ghajar, J. Visual tracking synchronization as a metric for concussion screening. J. Head Trauma Rehabil. 2010, 25, 293–305. [Google Scholar] [CrossRef]
- Kraus, M.F.; Susmaras, T.; Caughlin, B.P.; Walker, C.J.; Sweeney, J.A.; Little, D.M. White matter integrity and cognition in chronic traumatic brain injury: A diffusion tensor imaging study. Brain 2007, 130, 2508–2519. [Google Scholar] [CrossRef]
- Niogi, S.N.; Mukherjee, P.; Ghajar, J.; Johnson, C.E.; Kolster, R.; Lee, H.; Suh, M.; Zimmerman, R.D.; Manley, G.T.; McCandliss, B.D. Structural dissociation of attentional control and memory in adults with and without mild traumatic brain injury. Brain 2008, 131, 3209–3221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pal, D.; Gupta, R.K.; Agarwal, S.; Yadav, A.; Ojha, B.K.; Awasthi, A.; Rathore, R.K.; Pandey, C.M.; Narayana, P.A. Diffusion tensor tractography indices in patients with frontal lobe injury and its correlation with neuropsychological tests. Clin. Neurol. Neurosurg. 2012, 114, 564–571. [Google Scholar] [CrossRef] [PubMed]
- Soeda, A.; Nakashima, T.; Okumura, A.; Kuwata, K.; Shinoda, J.; Iwama, T. Cognitive impairment after traumatic brain injury: A functional magnetic resonance imaging study using the Stroop task. Neuroradiology 2005, 47, 501–506. [Google Scholar] [CrossRef]
- Mayer, A.R.; Mannell, M.V.; Ling, J.; Elgie, R.; Gasparovic, C.; Phillips, J.P.; Doezema, D.; Yeo, R.A. Auditory orienting and inhibition of return in mild traumatic brain injury: A FMRI study. Hum. Brain Mapp. 2009, 30, 4152–4166. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Whyte, J.; Patel, S.; Europa, E.; Slattery, J.; Coslett, H.B.; Detre, J.A. A perfusion fMRI study of the neural correlates of sustained-attention and working-memory deficits in chronic traumatic brain injury. Neurorehabil. Neural. Repair 2012, 26, 870–880. [Google Scholar] [CrossRef]
- Hibino, S.; Mase, M.; Shirataki, T.; Nagano, Y.; Fukagawa, K.; Abe, A.; Nishide, Y.; Aizawa, A.; Iida, A.; Ogawa, T.; et al. Oxyhemoglobin changes during cognitive rehabilitation after traumatic brain injury using near infrared spectroscopy. Neurol. Med. Chir. 2013, 53, 299–303. [Google Scholar] [CrossRef] [Green Version]
- Petley, L.; Bardouille, T.; Chiasson, D.; Froese, P.; Patterson, S.; Newman, A.; Omisade, A.; Beyea, S. Attentional dysfunction and recovery in concussion: Effects on the P300m and contingent magnetic variation. Brain Inj. 2018, 32, 464–473. [Google Scholar] [CrossRef]
- Bonnelle, V.; Leech, R.; Kinnunen, K.M.; Ham, T.E.; Beckmann, C.F.; De Boissezon, X.; Greenwood, R.J.; Sharp, D.J. Default mode network connectivity predicts sustained attention deficits after traumatic brain injury. J. Neurosci. 2011, 31, 13442–13451. [Google Scholar] [CrossRef] [PubMed]
- Shumskaya, E.; van Gerven, M.A.; Norris, D.G.; Vos, P.E.; Kessels, R.P. Abnormal connectivity in the sensorimotor network predicts attention deficits in traumatic brain injury. Exp. Brain Res. 2017, 235, 799–807. [Google Scholar] [CrossRef] [Green Version]
- Dunkley, B.T.; Da Costa, L.; Bethune, A.; Jetly, R.; Pang, E.W.; Taylor, M.J.; Doesburg, S.M. Low-frequency connectivity is associated with mild traumatic brain injury. Neuroimage Clin. 2015, 7, 611–621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Z.; Mazzola, C.A.; Catania, L.; Owoeye, O.; Yaramothu, C.; Alvarez, T.; Gao, Y.; Li, X. Altered cortical activation and connectivity patterns for visual attention processing in young adults post-traumatic brain injury: A functional near infrared spectroscopy study. CNS Neurosci. Ther. 2018, 24, 539–548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gawryluk, J.R.; Mazerolle, E.L.; D’Arcy, R.C. Does functional MRI detect activation in white matter? A review of emerging evidence, issues, and future directions. Front. Neurosci. 2014, 8, 239. [Google Scholar] [CrossRef] [Green Version]
- Mazerolle, E.L.; D’Arcy, R.C.; Beyea, S.D. Detecting functional magnetic resonance imaging activation in white matter: Interhemispheric transfer across the corpus callosum. BMC Neurosci. 2008, 9, 84. [Google Scholar] [CrossRef]
- Yarkoni, T.; Barch, D.M.; Gray, J.R.; Conturo, T.E.; Braver, T.S. BOLD correlates of trial-by-trial reaction time variability in gray and white matter: A multi-study fMRI analysis. PLoS ONE 2009, 4, e4257. [Google Scholar] [CrossRef] [Green Version]
- Fabri, M.; Polonara, G.; Mascioli, G.; Salvolini, U.; Manzoni, T. Topographical organization of human corpus callosum: An fMRI mapping study. Brain Res. 2011, 1370, 99–111. [Google Scholar] [CrossRef] [PubMed]
- Gawryluk, J.R.; Mazerolle, E.L.; Brewer, K.D.; Beyea, S.D.; D’Arcy, R.C. Investigation of fMRI activation in the internal capsule. BMC Neurosci. 2011, 12, 56. [Google Scholar] [CrossRef] [Green Version]
- Fabri, M.; Polonara, G. Functional topography of human corpus callosum: An FMRI mapping study. Neural Plast. 2013, 2013, 251308. [Google Scholar] [CrossRef] [Green Version]
- Han, K.; Chapman, S.B.; Krawczyk, D.C. Disrupted Intrinsic Connectivity among Default, Dorsal Attention, and Frontoparietal Control Networks in Individuals with Chronic Traumatic Brain Injury. J. Int. Neuropsychol. Soc. 2016, 22, 263–279. [Google Scholar] [CrossRef] [Green Version]
- Pandit, A.S.; Expert, P.; Lambiotte, R.; Bonnelle, V.; Leech, R.; Turkheimer, F.E.; Sharp, D.J. Traumatic brain injury impairs small-world topology. Neurology 2013, 80, 1826–1833. [Google Scholar] [CrossRef] [Green Version]
- Messe, A.; Caplain, S.; Pelegrini-Issac, M.; Blancho, S.; Levy, R.; Aghakhani, N.; Montreuil, M.; Benali, H.; Lehericy, S. Specific and evolving resting-state network alterations in post-concussion syndrome following mild traumatic brain injury. PLoS ONE 2013, 8, e65470. [Google Scholar] [CrossRef] [Green Version]
- Yan, H.; Feng, Y.; Wang, Q. Altered Effective Connectivity of Hippocampus-Dependent Episodic Memory Network in mTBI Survivors. Neural Plast. 2016, 2016, 6353845. [Google Scholar] [CrossRef]
- Jia, X.; Chang, X.; Bai, L.; Wang, Y.; Dong, D.; Gan, S.; Wang, S.; Li, X.; Yang, X.; Sun, Y.; et al. A Longitudinal Study of White Matter Functional Network in Mild Traumatic Brain Injury. J. Neurotrauma 2021, 38, 2686–2697. [Google Scholar] [CrossRef] [PubMed]
- Meier, T.B.; Bergamino, M.; Bellgowan, P.S.; Teague, T.K.; Ling, J.M.; Jeromin, A.; Mayer, A.R. Longitudinal assessment of white matter abnormalities following sports-related concussion. Hum. Brain Mapp. 2016, 37, 833–845. [Google Scholar] [CrossRef] [PubMed]
- Cubon, V.A.; Putukian, M.; Boyer, C.; Dettwiler, A. A diffusion tensor imaging study on the white matter skeleton in individuals with sports-related concussion. J. Neurotrauma 2011, 28, 189–201. [Google Scholar] [CrossRef] [PubMed]
- Chamard, E.; Lassonde, M.; Theoret, H. Neurometabolic, electrophysiological, and imaging abnormalities. Prog. Neurol. Surg. 2014, 28, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Stave, E.A.; De Bellis, M.D.; Hooper, S.R.; Woolley, D.P.; Chang, S.K.; Chen, S.D. Dimensions of Attention Associated With the Microstructure of Corona Radiata White Matter. J. Child Neurol. 2017, 32, 458–466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wright, N.F.; Vann, S.D.; Aggleton, J.P.; Nelson, A.J. A critical role for the anterior thalamus in directing attention to task-relevant stimuli. J. Neurosci. 2015, 35, 5480–5488. [Google Scholar] [CrossRef] [Green Version]
- Chiang, H.L.; Chen, Y.J.; Lo, Y.C.; Tseng, W.Y.; Gau, S.S. Altered white matter tract property related to impaired focused attention, sustained attention, cognitive impulsivity and vigilance in attention-deficit/ hyperactivity disorder. J. Psychiatry Neurosci. 2015, 40, 325–335. [Google Scholar] [CrossRef] [Green Version]
- Klarborg, B.; Skak Madsen, K.; Vestergaard, M.; Skimminge, A.; Jernigan, T.L.; Baare, W.F. Sustained attention is associated with right superior longitudinal fasciculus and superior parietal white matter microstructure in children. Hum. Brain Mapp. 2013, 34, 3216–3232. [Google Scholar] [CrossRef] [Green Version]
- Epstein, J.N.; Johnson, D.; Conners, C.K. Conners’ Adult ADHD Diagnostic Interview for DSM-IV; Multi-Health Systems: North Tonawanda, NY, USA, 2006. [Google Scholar]
- Oldfield, R.C. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 1971, 9, 97–113. [Google Scholar] [CrossRef]
- Barkley, R.A.; DuPaul, G.J.; McMurray, M.B. Comprehensive evaluation of attention deficit disorder with and without hyperactivity as defined by research criteria. J. Consult. Clin. Psychol. 1990, 58, 775. [Google Scholar] [CrossRef]
- Halperin, J.M.; O’Brien, J.D.; Newcorn, J.H.; Healey, J.M.; Pascualvaca, D.M.; Wolf, L.E.; Young, J.G. Validation of hyperactive, aggressive, and mixed hyperactive/aggressive childhood disorders: A research note. J. Child Psychol. Psychiatry 1990, 31, 455–459. [Google Scholar] [CrossRef]
- Tana, M.; Montin, E.; Cerutti, S.; Bianchi, A.M. Exploring cortical attentional system by using fMRI during a Continuous Perfomance Test. Comput. Intell. Neurosci. 2010, 2010, 329213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Sroubek, A.; Kelly, M.S.; Lesser, I.; Sussman, E.; He, Y.; Branch, C.; Foxe, J.J. Atypical pulvinar-cortical pathways during sustained attention performance in children with attention-deficit/hyperactivity disorder. J. Am. Acad. Child Adolesc. Psychiatry 2012, 51, 1197–1207.e1194. [Google Scholar] [CrossRef] [Green Version]
- Xia, S.; Foxe, J.J.; Sroubek, A.E.; Branch, C.; Li, X. Topological organization of the “small-world” visual attention network in children with attention deficit/hyperactivity disorder (ADHD). Front. Hum. Neurosci. 2014, 8, 162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jenkinson, M.; Bannister, P.; Brady, M.; Smith, S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage 2002, 17, 825–841. [Google Scholar] [CrossRef]
- Jenkinson, M.; Smith, S. A global optimisation method for robust affine registration of brain images. Med. Image Anal. 2001, 5, 143–156. [Google Scholar] [CrossRef]
- Smith, S.M.; Jenkinson, M.; Woolrich, M.W.; Beckmann, C.F.; Behrens, T.E.; Johansen-Berg, H.; Bannister, P.R.; De Luca, M.; Drobnjak, I.; Flitney, D.E.; et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 2004, 23 (Suppl. S1), S208–S219. [Google Scholar] [CrossRef] [Green Version]
- Woolrich, M.W.; Ripley, B.D.; Brady, M.; Smith, S.M. Temporal autocorrelation in univariate linear modeling of FMRI data. Neuroimage 2001, 14, 1370–1386. [Google Scholar] [CrossRef] [Green Version]
- Pang, J.C.; Robinson, P.A. Power spectrum of resting-state blood-oxygen-level-dependent signal. Phys. Rev. E 2019, 100, 022418. [Google Scholar] [CrossRef]
- Duff, E.P.; Johnston, L.A.; Xiong, J.; Fox, P.T.; Mareels, I.; Egan, G.F. The power of spectral density analysis for mapping endogenous BOLD signal fluctuations. Hum. Brain Mapp. 2008, 29, 778–790. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Gao, Y.; Ding, Z.; Gore, J.C. Power spectra reveal distinct BOLD resting-state time courses in white matter. Proc. Natl. Acad. Sci. USA 2021, 118, e2103104118. [Google Scholar] [CrossRef]
- Mori, S.; Oishi, K.; Jiang, H.; Jiang, L.; Li, X.; Akhter, K.; Hua, K.; Faria, A.V.; Mahmood, A.; Woods, R.; et al. Stereotaxic white matter atlas based on diffusion tensor imaging in an ICBM template. Neuroimage 2008, 40, 570–582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, S.; Zhang, Y. Fourier transform power spectrum is a potential measure of tissue alignment in standard MRI: A multiple sclerosis study. PLoS ONE 2017, 12, e0175979. [Google Scholar] [CrossRef] [PubMed]
- Perge, J.A.; Niven, J.E.; Mugnaini, E.; Balasubramanian, V.; Sterling, P. Why do axons differ in caliber? J. Neurosci. 2012, 32, 626–638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mallat, S. A Wavelet Tour of Signal Processing; Academic Press: Cambridge, MA, USA, 1998. [Google Scholar]
- Achard, S.; Salvador, R.; Whitcher, B.; Suckling, J.; Bullmore, E. A resilient, low-frequency, small-world human brain functional network with highly connected association cortical hubs. J. Neurosci. 2006, 26, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Bassett, D.S.; Wymbs, N.F.; Porter, M.A.; Mucha, P.J.; Carlson, J.M.; Grafton, S.T. Dynamic reconfiguration of human brain networks during learning. Proc. Natl. Acad. Sci. USA 2011, 108, 7641–7646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ginestet, C.E.; Simmons, A. Statistical parametric network analysis of functional connectivity dynamics during a working memory task. Neuroimage 2011, 55, 688–704. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xia, S.; Bertisch, H.C.; Branch, C.A.; Delisi, L.E. Unique topology of language processing brain network: A systems-level biomarker of schizophrenia. Schizophr. Res. 2012, 141, 128–136. [Google Scholar] [CrossRef] [Green Version]
- Latora, V.; Marchiori, M. Efficient behavior of small-world networks. Phys. Rev. Lett. 2001, 87, 198701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Achard, S.; Bullmore, E. Efficiency and cost of economical brain functional networks. PLoS Comput. Biol. 2007, 3, e17. [Google Scholar] [CrossRef]
- Bullmore, E.; Sporns, O. Complex brain networks: Graph theoretical analysis of structural and functional systems. Nat. Rev. Neurosci. 2009, 10, 186–198. [Google Scholar] [CrossRef]
- Watts, D.J.; Strogatz, S.H. Collective dynamics of ’small-world’ networks. Nature 1998, 393, 440–442. [Google Scholar] [CrossRef]
- Sporns, O.; Honey, C.J.; Kotter, R. Identification and classification of hubs in brain networks. PLoS ONE 2007, 2, e1049. [Google Scholar] [CrossRef] [PubMed]
- Onnela, J.P.; Saramaki, J.; Kertesz, J.; Kaski, K. Intensity and coherence of motifs in weighted complex networks. Phys. Rev. E Stat. Nonlin. Soft Matter. Phys. 2005, 71, 065103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, L.; Li, H.; Zhuo, J.; Zhang, Y.; Wang, J.; Chen, L.; Yang, Z.; Chu, C.; Xie, S.; Laird, A.R.; et al. The Human Brainnetome Atlas: A New Brain Atlas Based on Connectional Architecture. Cereb. Cortex 2016, 26, 3508–3526. [Google Scholar] [CrossRef]
- Power, J.D.; Cohen, A.L.; Nelson, S.M.; Wig, G.S.; Barnes, K.A.; Church, J.A.; Vogel, A.C.; Laumann, T.O.; Miezin, F.M.; Schlaggar, B.L.; et al. Functional network organization of the human brain. Neuron 2011, 72, 665–678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wig, G.S.; Laumann, T.O.; Cohen, A.L.; Power, J.D.; Nelson, S.M.; Glasser, M.F.; Miezin, F.M.; Snyder, A.Z.; Schlaggar, B.L.; Petersen, S.E. Parcellating an individual subject’s cortical and subcortical brain structures using snowball sampling of resting-state correlations. Cereb. Cortex 2014, 24, 2036–2054. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dunn, O.J. Multiple Comparisons among Means. J. Am. Stat. Assoc. 1961, 56, 52–64. [Google Scholar] [CrossRef]
- Rubinov, M.; Sporns, O. Complex network measures of brain connectivity: Uses and interpretations. Neuroimage 2010, 52, 1059–1069. [Google Scholar] [CrossRef] [PubMed]
- Emos, M.C.; Agarwal, S. Neuroanatomy, Internal Capsule. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Nicole, M.; Gage, B.J.B. Fundamentals of Cognitive Neuroscience, 2nd ed.; Academic Press: Cambridge, MA, USA, 2018. [Google Scholar]
- George, K.; Das, J.M. Neuroanatomy, Thalamocortical Radiations. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2021.
- Qiu, M.G.; Ye, Z.; Li, Q.Y.; Liu, G.J.; Xie, B.; Wang, J. Changes of brain structure and function in ADHD children. Brain Topogr. 2011, 24, 243–252. [Google Scholar] [CrossRef]
- Nagel, B.J.; Bathula, D.; Herting, M.; Schmitt, C.; Kroenke, C.D.; Fair, D.; Nigg, J.T. Altered white matter microstructure in children with attention-deficit/hyperactivity disorder. J. Am. Acad. Child Adolesc. Psychiatry 2011, 50, 283–292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pavuluri, M.N.; Yang, S.; Kamineni, K.; Passarotti, A.M.; Srinivasan, G.; Harral, E.M.; Sweeney, J.A.; Zhou, X.J. Diffusion tensor imaging study of white matter fiber tracts in pediatric bipolar disorder and attention-deficit/hyperactivity disorder. Biol. Psychiatry 2009, 65, 586–593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kobel, M.; Bechtel, N.; Specht, K.; Klarhofer, M.; Weber, P.; Scheffler, K.; Opwis, K.; Penner, I.K. Structural and functional imaging approaches in attention deficit/hyperactivity disorder: Does the temporal lobe play a key role? Psychiatry Res. 2010, 183, 230–236. [Google Scholar] [CrossRef]
- Scheibel, R.S.; Newsome, M.R.; Steinberg, J.L.; Pearson, D.A.; Rauch, R.A.; Mao, H.; Troyanskaya, M.; Sharma, R.G.; Levin, H.S. Altered brain activation during cognitive control in patients with moderate to severe traumatic brain injury. Neurorehabil. Neural Repair 2007, 21, 36–45. [Google Scholar] [CrossRef]
- Turner, G.R.; Levine, B. Augmented neural activity during executive control processing following diffuse axonal injury. Neurology 2008, 71, 812–818. [Google Scholar] [CrossRef] [Green Version]
- Salmond, C.H.; Chatfield, D.A.; Menon, D.K.; Pickard, J.D.; Sahakian, B.J. Cognitive sequelae of head injury: Involvement of basal forebrain and associated structures. Brain 2005, 128, 189–200. [Google Scholar] [CrossRef] [Green Version]
- Little, D.M.; Kraus, M.F.; Joseph, J.; Geary, E.K.; Susmaras, T.; Zhou, X.J.; Pliskin, N.; Gorelick, P.B. Thalamic integrity underlies executive dysfunction in traumatic brain injury. Neurology 2010, 74, 558–564. [Google Scholar] [CrossRef] [Green Version]
- Grossman, E.J.; Ge, Y.; Jensen, J.H.; Babb, J.S.; Miles, L.; Reaume, J.; Silver, J.M.; Grossman, R.I.; Inglese, M. Thalamus and cognitive impairment in mild traumatic brain injury: A diffusional kurtosis imaging study. J. Neurotrauma 2012, 29, 2318–2327. [Google Scholar] [CrossRef]
- Grossman, E.J.; Jensen, J.H.; Babb, J.S.; Chen, Q.; Tabesh, A.; Fieremans, E.; Xia, D.; Inglese, M.; Grossman, R.I. Cognitive impairment in mild traumatic brain injury: A longitudinal diffusional kurtosis and perfusion imaging study. AJNR Am. J. Neuroradiol. 2013, 34, 951–957, S951-953. [Google Scholar] [CrossRef] [Green Version]
- Vargo, J.M.; Grachek, R.A.; Rockswold, G.L. Light deprivation soon after frontal brain trauma accelerates recovery from attentional deficits and promotes functional normalization of basal ganglia. J. Trauma 1999, 47, 265–272, discussion 273–264. [Google Scholar] [CrossRef]
- O’Phelan, K.H.; Otoshi, C.K.; Ernst, T.; Chang, L. Common Patterns of Regional Brain Injury Detectable by Diffusion Tensor Imaging in Otherwise Normal-Appearing White Matter in Patients with Early Moderate to Severe Traumatic Brain Injury. J. Neurotrauma 2018, 35, 739–749. [Google Scholar] [CrossRef]
- Churchill, N.W.; Caverzasi, E.; Graham, S.J.; Hutchison, M.G.; Schweizer, T.A. White matter during concussion recovery: Comparing diffusion tensor imaging (DTI) and neurite orientation dispersion and density imaging (NODDI). Hum. Brain Mapp. 2019, 40, 1908–1918. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lindemer, E.R.; Salat, D.H.; Leritz, E.C.; McGlinchey, R.E.; Milberg, W.P. Reduced cortical thickness with increased lifetime burden of PTSD in OEF/OIF Veterans and the impact of comorbid TBI. Neuroimage Clin. 2013, 2, 601–611. [Google Scholar] [CrossRef] [Green Version]
- Ogren, J.A.; Tripathi, R.; Macey, P.M.; Kumar, R.; Stern, J.M.; Eliashiv, D.S.; Allen, L.A.; Diehl, B.; Engel, J., Jr.; Rani, M.R.S.; et al. Regional cortical thickness changes accompanying generalized tonic-clonic seizures. Neuroimage Clin. 2018, 20, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Tate, D.F.; York, G.E.; Reid, M.W.; Cooper, D.B.; Jones, L.; Robin, D.A.; Kennedy, J.E.; Lewis, J. Preliminary findings of cortical thickness abnormalities in blast injured service members and their relationship to clinical findings. Brain Imaging Behav. 2014, 8, 102–109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antonakakis, M.; Dimitriadis, S.I.; Zervakis, M.; Papanicolaou, A.C.; Zouridakis, G. Altered Rich-Club and Frequency-Dependent Subnetwork Organization in Mild Traumatic Brain Injury: A MEG Resting-State Study. Front. Hum. Neurosci. 2017, 11, 416. [Google Scholar] [CrossRef]
- Antonakakis, M.; Dimitriadis, S.I.; Zervakis, M.; Papanicolaou, A.C.; Zouridakis, G. Aberrant Whole-Brain Transitions and Dynamics of Spontaneous Network Microstates in Mild Traumatic Brain Injury. Front. Comput. Neurosci. 2019, 13, 90. [Google Scholar] [CrossRef] [Green Version]
- Colvin, A.C.; Mullen, J.; Lovell, M.R.; West, R.V.; Collins, M.W.; Groh, M. The role of concussion history and gender in recovery from soccer-related concussion. Am. J. Sports Med. 2009, 37, 1699–1704. [Google Scholar] [CrossRef]
- Covassin, T.; Schatz, P.; Swanik, C.B. Sex differences in neuropsychological function and post-concussion symptoms of concussed collegiate athletes. Neurosurgery 2007, 61, 345–350, discussion 350–341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Covassin, T.; Elbin, R.J.; Harris, W.; Parker, T.; Kontos, A. The role of age and sex in symptoms, neurocognitive performance, and postural stability in athletes after concussion. Am. J. Sports Med. 2012, 40, 1303–1312. [Google Scholar] [CrossRef] [PubMed]
- Covassin, T.; Elbin, R.J.; Bleecker, A.; Lipchik, A.; Kontos, A.P. Are there differences in neurocognitive function and symptoms between male and female soccer players after concussions? Am. J. Sports Med. 2013, 41, 2890–2895. [Google Scholar] [CrossRef] [PubMed]
- Fakhran, S.; Yaeger, K.; Collins, M.; Alhilali, L. Sex differences in white matter abnormalities after mild traumatic brain injury: Localization and correlation with outcome. Radiology 2014, 272, 815–823. [Google Scholar] [CrossRef]
- McGlade, E.; Rogowska, J.; Yurgelun-Todd, D. Sex differences in orbitofrontal connectivity in male and female veterans with TBI. Brain Imaging Behav. 2015, 9, 535–549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goodrich-Hunsaker, N.J.; Abildskov, T.J.; Black, G.; Bigler, E.D.; Cohen, D.M.; Mihalov, L.K.; Bangert, B.A.; Taylor, H.G.; Yeates, K.O. Age- and sex-related effects in children with mild traumatic brain injury on diffusion magnetic resonance imaging properties: A comparison of voxelwise and tractography methods. J. Neurosci. Res. 2018, 96, 626–641. [Google Scholar] [CrossRef]
- Matser, J.T.; Kessels, A.G.; Lezak, M.D.; Troost, J. A dose-response relation of headers and concussions with cognitive impairment in professional soccer players. J. Clin. Exp. Neuropsychol. 2001, 23, 770–774. [Google Scholar] [CrossRef]
- Tysvaer, A.T.; Lochen, E.A. Soccer injuries to the brain. A neuropsychologic study of former soccer players. Am. J. Sports Med. 1991, 19, 56–60. [Google Scholar] [CrossRef]
- Matser, J.T.; Kessels, A.G.; Jordan, B.D.; Lezak, M.D.; Troost, J. Chronic traumatic brain injury in professional soccer players. Neurology 1998, 51, 791–796. [Google Scholar] [CrossRef]
- Kontos, A.P.; Dolese, A.; Elbin, R.J.; Covassin, T.; Warren, B.L. Relationship of soccer heading to computerized neurocognitive performance and symptoms among female and male youth soccer players. Brain Inj. 2011, 25, 1234–1241. [Google Scholar] [CrossRef] [PubMed]
- Kaminski, T.W.; Wikstrom, A.M.; Gutierrez, G.M.; Glutting, J.J. Purposeful heading during a season does not influence cognitive function or balance in female soccer players. J. Clin. Exp. Neuropsychol. 2007, 29, 742–751. [Google Scholar] [CrossRef] [PubMed]
- Kaminski, T.W.; Cousino, E.S.; Glutting, J.J. Examining the relationship between purposeful heading in soccer and computerized neuropsychological test performance. Res. Q Exerc. Sport 2008, 79, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Siegel, J.S.; Shulman, G.L.; Corbetta, M. Measuring functional connectivity in stroke: Approaches and considerations. J. Cereb. Blood Flow Metab. 2017, 37, 2665–2678. [Google Scholar] [CrossRef] [Green Version]



| NC (N = 43) | TBI (N = 42) | ||
|---|---|---|---|
| Mean (SD) | Mean (SD) | p Value | |
| Age | 22.36 (2.74) | 21.63 (2.00) | 0.167 |
| Education year | 14.98 (1.95) | 14.26 (1.56) | 0.066 |
| Mother’s education year | 15.35 (2.20) | 15.55 (2.70) | 0.710 |
| Father’s education year | 15.77 (2.81) | 15.50 (2.78) | 0.660 |
| CAARS scores | |||
| Inattentive raw scores | 4.67 (2.81) | 9.31 (6.28) | <0.001 |
| Inattentive T-scores | 45.88 (6.48) | 57.02 (15.18) | <0.001 |
| Hyperactive/impulsive raw scores | 5.07 (2.76) | 9.19 (5.80) | <0.001 |
| Hyperactive/impulsive T-scores | 42.58 (5.93) | 52.52 (14.66) | <0.001 |
| N (%) | N (%) | p Value | |
| Male | 23 (53.49) | 21 (50.00) | 0.917 |
| Right-handed | 43 (100) | 42 (100) | 1.000 |
| Race/Ethnicity | 0.094 | ||
| Caucasian | 12 (27.91) | 21 (50.00) | |
| Black or African American | 4 (9.30) | 7 (2.38) | |
| Asian | 20 (46.51) | 9 (21.43) | |
| Hispanic/Latino | 2 (4.65) | 2 (4.76) | |
| More than one race | 5 (11.63) | 3 (7.14) | |
| Functional MRI task performance measures | Mean (SD) | Mean (SD) | p Value |
| Accuracy rate | 0.99 (0.04) | 0.99 (0.01) | 0.344 |
| Omission error rate | 0.009 (0.03) | 0.001 (0.005) | 0.131 |
| Commission error rate | 0.003 (0.01) | 0.005 (0.01) | 0.464 |
| Overall response reaction time (ms) | 607.09 (134.73) | 604.45 (132.83) | 0.928 |
| Correct response reaction time(ms) | 606.72 (135.02) | 603.83 (132.47) | 0.921 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Z.; Cao, M.; Di, X.; Wu, K.; Gao, Y.; Li, X. Regional Topological Aberrances of White Matter- and Gray Matter-Based Functional Networks for Attention Processing May Foster Traumatic Brain Injury-Related Attention Deficits in Adults. Brain Sci. 2022, 12, 16. https://doi.org/10.3390/brainsci12010016
Wu Z, Cao M, Di X, Wu K, Gao Y, Li X. Regional Topological Aberrances of White Matter- and Gray Matter-Based Functional Networks for Attention Processing May Foster Traumatic Brain Injury-Related Attention Deficits in Adults. Brain Sciences. 2022; 12(1):16. https://doi.org/10.3390/brainsci12010016
Chicago/Turabian StyleWu, Ziyan, Meng Cao, Xin Di, Kai Wu, Yu Gao, and Xiaobo Li. 2022. "Regional Topological Aberrances of White Matter- and Gray Matter-Based Functional Networks for Attention Processing May Foster Traumatic Brain Injury-Related Attention Deficits in Adults" Brain Sciences 12, no. 1: 16. https://doi.org/10.3390/brainsci12010016