An Overview on Cognitive Function Enhancement through Physical Exercises
Abstract
:1. Introduction
2. Types of Physical Activities That Enhance Cognitive Functions
2.1. Aerobic Exercise
2.2. Resistance Training
2.3. Sports
2.4. Dance
2.5. Yoga
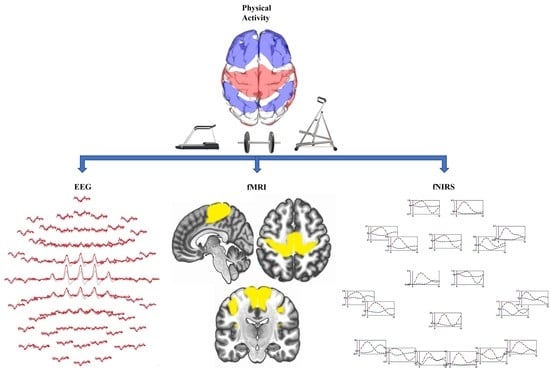
3. Neuroimaging Modalities
3.1. Functional Magnetic Resonance Imaging (fMRI)
3.2. Functional near Infrared Spectroscopy (fNIRS)
3.3. Electroencephalography (EEG)
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dosenbach, N.U.; Fair, D.A.; Miezin, F.M.; Cohen, A.L.; Wenger, K.K.; Dosenbach, R.A.; Fox, M.D.; Snyder, A.Z.; Vincent, J.L.; Raichle, M.E. Distinct brain networks for adaptive and stable task control in humans. Proc. Natl. Acad. Sci. USA 2007, 104, 11073–11078. [Google Scholar] [CrossRef] [Green Version]
- Green, M.F.; Horan, W.P.; Lee, J. Nonsocial and social cognition in schizophrenia: Current evidence and future directions. World Psychiatry 2019, 18, 146–161. [Google Scholar] [CrossRef] [Green Version]
- Lv, M.; Liu, H.; Zhou, W.; Zheng, C. Efficiency model of micro-course study based on cognitive psychology in the college. Comput. Hum. Behav. 2020, 107, 106027. [Google Scholar] [CrossRef]
- Cumming, T.B.; Brodtmann, A.; Darby, D.; Bernhardt, J. The importance of cognition to quality of life after stroke. J. Psychosom. Res. 2014, 77, 374–379. [Google Scholar] [CrossRef] [PubMed]
- Bowie, C.R.; Harvey, P.D. Cognition in schizophrenia: Impairments, determinants, and functional importance. Psychiatr. Clin. 2005, 28, 613–633. [Google Scholar] [CrossRef]
- Javed, A.; Charles, A. The importance of social cognition in improving functional outcomes in schizophrenia. Front. Psychiatry 2018, 9, 157. [Google Scholar] [CrossRef] [PubMed]
- Teachman, B.A.; Cody, M.W.; Clerkin, E.M. Clinical Applications of Implicit Social Cognition Theories and Methods; The Guilford Press: New York, NY, USA, 2010. [Google Scholar]
- Zona, C.; Raab, M.; Fischer, M.H. Embodied perspectives on behavioral cognitive enhancement. J. Cogn. Enhanc. 2019, 3, 144–160. [Google Scholar] [CrossRef] [Green Version]
- Dubljević, V.; Venero, C.; Knafo, S. What is cognitive enhancement? In Cognitive Enhancement; Elsevier: Amsterdam, The Netherlands, 2015; pp. 1–9. [Google Scholar]
- Weisberg, S.M.; Newcombe, N.S. Embodied cognition and STEM learning: Overview of a topical collection in CR: PI. Cogn. Res. Princ. Implic. 2017, 2, 38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carlson, R.A.; Avraamides, M.N.; Cary, M.; Strasberg, S. What do the hands externalize in simple arithmetic? J. Exp. Psychol. Learn. Mem. Cogn. 2007, 33, 747. [Google Scholar] [CrossRef]
- Sandi, C. Stress and cognition. Wiley Interdiscip. Rev. Cogn. Sci. 2013, 4, 245–261. [Google Scholar] [CrossRef]
- Dahl, R.E. The Impact of Inadequate Sleep On Children’s Daytime Cognitive Function. Semin. Pediatr. Neurol. 1996, 3, 44–50. [Google Scholar] [CrossRef]
- Jang, A.R.; Yoon, J.Y. Factors affecting reversion from mild cognitive impairment to normal cognition in midlife to later life in Korea: A national population study. Geriatr. Gerontol. Int. 2019, 19, 1129–1135. [Google Scholar] [CrossRef]
- Harris, S.E.; Deary, I.J. The genetics of cognitive ability and cognitive ageing in healthy older people. Trends Cogn. Sci. 2011, 15, 388–394. [Google Scholar] [CrossRef]
- Kim, M.; Park, J.-M. Factors affecting cognitive function according to gender in community-dwelling elderly individuals. Epidemiol. Health 2017, 39, e2017054. [Google Scholar] [CrossRef] [Green Version]
- Wells, N.M. At home with nature: Effects of “greenness” on children’s cognitive functioning. Environ. Behav. 2000, 32, 775–795. [Google Scholar] [CrossRef] [Green Version]
- Bellisle, F. Effects of diet on behaviour and cognition in children. Br. J. Nutr. 2004, 92, S227–S232. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.J.; Cha, E.J.; Kim, S.M.; Kang, K.D.; Han, D.H. The effects of taekwondo training on brain connectivity and body intelligence. Psychiatry Investig. 2015, 12, 335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baune, B.T.; Renger, L. Pharmacological and non-pharmacological interventions to improve cognitive dysfunction and functional ability in clinical depression–a systematic review. Psychiatry Res. 2014, 219, 25–50. [Google Scholar] [CrossRef] [PubMed]
- Clark, G.J.F.; Schlabach, T.L. Systematic review of occupational therapy interventions to improve cognitive development in children ages birth–5 years. Am. J. Occup. Ther. 2013, 67, 425–430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seidler, R.D.; Bernard, J.A.; Buschkuehl, M.; Jaeggi, S.; Jonides, J.; Humfleet, J. Cognitive Training as an Intervention to Improve Driving Ability in the Older Adult; Michigan Center for Advancing Safe Transportation Throughout the Lifespan: Ann Arbor, MI, USA, 2010.
- Kray, J.; Ferdinand, N.K. How to improve cognitive control in development during childhood: Potentials and limits of cognitive interventions. Child Dev. Perspect. 2013, 7, 121–125. [Google Scholar] [CrossRef]
- Huntley, J.; Gould, R.; Liu, K.; Smith, M.; Howard, R. Do cognitive interventions improve general cognition in dementia? A meta-analysis and meta-regression. BMJ Open 2015, 5, e005247. [Google Scholar] [CrossRef] [Green Version]
- Dietz, P. The influence of sports on cognitive task performance—A critical overview. Cogn. Enhanc. 2013, 67–72. [Google Scholar]
- Lautenschlager, N.T.; Almeida, O.P. Physical activity and cognition in old age. Curr. Opin. Psychiatry 2006, 19, 190–193. [Google Scholar] [CrossRef] [PubMed]
- Kramer, A.F.; Erickson, K.I. Capitalizing on cortical plasticity: Influence of physical activity on cognition and brain function. Trends Cogn. Sci. 2007, 11, 342–348. [Google Scholar] [CrossRef]
- Esteban-Cornejo, I.; Tejero-Gonzalez, C.M.; Sallis, J.F.; Veiga, O.L. Physical activity and cognition in adolescents: A systematic review. J. Sci. Med. Sport 2015, 18, 534–539. [Google Scholar] [CrossRef] [PubMed]
- Bademli, K.; Lok, N.; Canbaz, M.; Lok, S. Effects of Physical Activity Program on cognitive function and sleep quality in elderly with mild cognitive impairment: A randomized controlled trial. Perspect. Psychiatr. Care 2019, 55, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Erickson, K.I.; Hillman, C.H.; Kramer, A.F. Physical activity, brain, and cognition. Curr. Opin. Behav. Sci. 2015, 4, 27–32. [Google Scholar] [CrossRef]
- Chu, C.-H.; Kramer, A.F.; Song, T.-F.; Wu, C.-H.; Hung, T.-M.; Chang, Y.-K. Acute exercise and neurocognitive development in preadolescents and young adults: An ERP study. Neural Plast. 2017, 2017, 2631909. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Men, W.-W.; Chang, Y.-K.; Fan, M.-X.; Ji, L.; Wei, G.-X. Acute aerobic exercise increases cortical activity during working memory: A functional MRI study in female college students. PLoS ONE 2014, 9, e99222. [Google Scholar] [CrossRef]
- Zhaang, Y.; Shi, W.; Wang, H.; Liu, M.; Tang, D. The impact of acute exercise on implicit cognitive reappraisal in association with left dorsolateral prefronta activation: A fNIRS study. Behav. Brain Res. 2021, 406, 113233. [Google Scholar] [CrossRef]
- Firth, J.; Stubbs, B.; Vancampfort, D.; Schuch, F.; Lagopoulos, J.; Rosenbaum, S.; Ward, P.B. Effect of aerobic exercise on hippocampal volume in humans: A systematic review and meta-analysis. NeuroImage 2018, 166, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Chaddock-Heyman, L.; Erickson, K.I.; Voss, M.; Knecht, A.; Pontifex, M.B.; Castelli, D.; Hillman, C.; Kramer, A. The effects of physical activity on functional MRI activation associated with cognitive control in children: A randomized controlled intervention. Front. Hum. Neurosci. 2013, 7, 72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gligoroska, J.P.; Manchevska, S. The effect of physical activity on cognition–physiological mechanisms. Mater. Socio-Med. 2012, 24, 198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colcombe, S.J.; Erickson, K.I.; Scalf, P.E.; Kim, J.S.; Prakash, R.; McAuley, E.; Elavsky, S.; Marquez, D.X.; Hu, L.; Kramer, A.F. Aerobic exercise training increases brain volume in aging humans. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2006, 61, 1166–1170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haeger, A.; Costa, A.S.; Schulz, J.B.; Reetz, K. Cerebral changes improved by physical activity during cognitive decline: A systematic review on MRI studies. NeuroImage Clin. 2019, 23, 101933. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.C.; Nielson, K.A.; Antuono, P.; Lyons, J.-A.; Hanson, R.J.; Butts, A.M.; Hantke, N.C.; Verber, M.D. Semantic memory functional MRI and cognitive function after exercise intervention in mild cognitive impairment. J. Alzheimer’s Dis. 2013, 37, 197–215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harveson, A.T.; Hannon, J.C.; Brusseau, T.A.; Podlog, L.; Papadopoulos, C.; Durrant, L.H.; Hall, M.S.; Kang, K.-d. Acute effects of 30 minutes resistance and aerobic exercise on cognition in a high school sample. Res. Q. Exerc. Sport 2016, 87, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Herold, F.; Behrendt, T.; Torpel, A.; Hamacher, D.; Muller, N.G.; Schega, L. Cortical hemodynamics as a function of handgrip strength and cognitive performance: A cross-sectional fNIRS study in younger adults. BMC Neurosci. 2021, 22, 10. [Google Scholar] [CrossRef]
- Best, J.R.; Chiu, B.K.; Hsu, C.L.; Nagamatsu, L.S.; Liu-Ambrose, T. Long-term effects of resistance exercise training on cognition and brain volume in older women: Results from a randomized controlled trial. J. Int. Neuropsychol. Soc. 2015, 21, 745–756. [Google Scholar] [CrossRef]
- Chang, Y.-K.; Pan, C.-Y.; Chen, F.-T.; Tsai, C.-L.; Huang, C.-C. Effect of resistance-exercise training on cognitive function in healthy older adults: A review. J. Aging Phys. Act. 2012, 20, 497–517. [Google Scholar] [CrossRef] [Green Version]
- Furlano, J.A.; Nagamatsu, L.S. Feasibility of a 6-month pilot randomised controlled trial of resistance training on cognition and brain health in Canadian older adults at-risk for diabetes: Study protocol. BMJ Open 2019, 9, e032047. [Google Scholar] [CrossRef]
- Suo, C.; Singh, M.F.; Gates, N.; Wen, W.; Sachdev, P.; Brodaty, H.; Saigal, N.; Wilson, G.C.; Meiklejohn, J.; Singh, N. Therapeutically relevant structural and functional mechanisms triggered by physical and cognitive exercise. Mol. Psychiatry 2016, 21, 1633–1642. [Google Scholar] [CrossRef] [Green Version]
- Bento-Torres, N.V.O.; Bento-Torres, J.; Tomás, A.M.; Souza, L.G.T.d.; Freitas, J.O.d.; Pantoja, J.A.d.S.; Picanço-Diniz, C.W. Water-based exercise and resistance training improve cognition in older adults. Rev. Bras. De Med. Do Esporte 2019, 25, 71–75. [Google Scholar] [CrossRef]
- Burke, D.; Al-Adawi, S.; Lee, Y.; Audette, J. Martial arts as sport and therapy. J. Sports Med. Phys. Fit. 2007, 47, 96. [Google Scholar]
- Douris, P.; Douris, C.; Balder, N.; LaCasse, M.; Rand, A.; Tarapore, F.; Zhuchkan, A.; Handrakis, J. Martial art training and cognitive performance in middle-aged adults. J. Hum. Kinet. 2015, 47, 277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berti, B.; Momi, D.; Sprugnoli, G.; Neri, F.; Bonifazi, M.; Rossi, A.; Muscettola, M.M.; Benocci, R.; Santarnecchi, E.; Rossi, S. Peculiarities of Functional Connectivity—Including Cross-Modal Patterns—In Professional Karate Athletes: Correlations with Cognitive and Motor Performances. Neural Plast. 2019, 2019, 6807978. [Google Scholar] [CrossRef]
- Alesi, M.; Bianco, A.; Padulo, J.; Vella, F.P.; Petrucci, M.; Paoli, A.; Palma, A.; Pepi, A. Motor and cognitive development: The role of karate. Muscles Ligaments Tendons J. 2014, 4, 114. [Google Scholar] [CrossRef] [PubMed]
- Lopes Filho, B.J.P.; Oliveira, C.R.d.; Gottlieb, M.G.V. Effects of karate-dŌ training in older adults cognition: Randomized controlled trial. J. Phys. Educ. 2019, 30. [Google Scholar] [CrossRef] [Green Version]
- Witte, K.; Kropf, S.; Darius, S.; Emmermacher, P.; Böckelmann, I. Comparing the effectiveness of karate and fitness training on cognitive functioning in older adults—A randomized controlled trial. J. Sport Health Sci. 2016, 5, 484–490. [Google Scholar] [CrossRef] [Green Version]
- Pons Van Dijk, G.; Lenssen, A.; Leffers, P.; Kingma, H.; Lodder, J. Taekwondo training improves balance in volunteers over 40. Front. Aging Neurosci. 2013, 5, 10. [Google Scholar] [CrossRef] [Green Version]
- Lakes, K.D.; Bryars, T.; Sirisinahal, S.; Salim, N.; Arastoo, S.; Emmerson, N.; Kang, D.; Shim, L.; Wong, D.; Kang, C.J. The healthy for life taekwondo pilot study: A preliminary evaluation of effects on executive function and BMI, feasibility, and acceptability. Ment. Health Phys. Act. 2013, 6, 181–188. [Google Scholar] [CrossRef] [Green Version]
- Fujiwara, H.; Ueno, T.; Yoshimura, S.; Kobayashi, K.; Miyagi, T.; Oishi, N.; Murai, T. Martial arts “Kendo” and the motivation network during attention processing: An fMRI study. Front. Hum. Neurosci. 2019, 13, 170. [Google Scholar] [CrossRef] [Green Version]
- Johnstone, A.; Marí-Beffa, P. The effects of martial arts training on attentional networks in typical adults. Front. Psychol. 2018, 9, 80. [Google Scholar] [CrossRef]
- Wang, C.-H.; Chang, C.-C.; Liang, Y.-M.; Shih, C.-M.; Chiu, W.-S.; Tseng, P.; Hung, D.L.; Tzeng, O.J.; Muggleton, N.G.; Juan, C.-H. Open vs. closed skill sports and the modulation of inhibitory control. PLoS ONE 2013, 8, e55773. [Google Scholar] [CrossRef] [Green Version]
- Balser, N.; Lorey, B.; Pilgramm, S.; Naumann, T.; Kindermann, S.; Stark, R.; Zentgraf, K.; Williams, A.M.; Munzert, J. The influence of expertise on brain activation of the action observation network during anticipation of tennis and volleyball serves. Front. Hum. Neurosci. 2014, 8, 568. [Google Scholar] [CrossRef] [Green Version]
- Balardin, J.B.; Zimeo Morais, G.A.; Furucho, R.A.; Trambaiolli, L.; Vanzella, P.; Biazoli, C., Jr.; Sato, J.R. Imaging brain function with functional near-infrared spectroscopy in unconstrained environments. Front. Hum. Neurosci. 2017, 11, 258. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Li, A.; Yu, L. “Neural efficiency” of athletes’ brain during visuo-spatial task: An fMRI study on table tennis players. Front. Behav. Neurosci. 2017, 11, 72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wright, M.J.; Bishop, D.T.; Jackson, R.C.; Abernethy, B. Functional MRI reveals expert-novice differences during sport-related anticipation. Neuroreport 2010, 21, 94–98. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Zeng, Y.; Zhang, L.; Wang, S.; Wang, D.; Tan, X.; Zhu, X.; Zhang, J. The role of visual perception in action anticipation in basketball athletes. Neuroscience 2013, 237, 29–41. [Google Scholar] [CrossRef]
- Tan, X.-Y.; Pi, Y.-L.; Wang, J.; Li, X.-P.; Zhang, L.-L.; Dai, W.; Zhu, H.; Ni, Z.; Zhang, J.; Wu, Y. Morphological and functional differences between athletes and novices in cortical neuronal networks. Front. Hum. Neurosci. 2017, 10, 660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rehfeld, K.; Lüders, A.; Hökelmann, A.; Lessmann, V.; Kaufmann, J.; Brigadski, T.; Müller, P.; Müller, N.G. Dance training is superior to repetitive physical exercise in inducing brain plasticity in the elderly. PLoS ONE 2018, 13, e0196636. [Google Scholar] [CrossRef] [Green Version]
- Quiroga Murcia, C.; Kreutz, G.; Clift, S.; Bongard, S. Shall we dance? An exploration of the perceived benefits of dancing on well-being. Arts Health 2010, 2, 149–163. [Google Scholar] [CrossRef]
- Froeliger, B.; Garland, E.L.; Modlin, L.A.; McClernon, F.J. Neurocognitive correlates of the effects of yoga meditation practice on emotion and cognition: A pilot study. Front. Integr. Neurosci. 2012, 6, 48. [Google Scholar] [CrossRef] [Green Version]
- Brown, S.; Martinez, M.J.; Parsons, L.M. The neural basis of human dance. Cereb. Cortex 2006, 16, 1157–1167. [Google Scholar] [CrossRef]
- Sejnoha Minsterova, A.; Klobusiakova, P.; Kropacova, S.; Novakova, L.; Brabenec, L.; Balazova, Z.; Grmela, R.; Skotakova, A.; Svobodova, L.; Rektorova, I. Multishell Diffusion MRI Reflects Improved Physical Fitness Induced by Dance Intervention. Neural Plast. 2020, 2020, 8836925. [Google Scholar] [CrossRef]
- Gothe, N.P.; Hayes, J.M.; Temali, C.; Damoiseaux, J.S. Differences in brain structure and function among yoga practitioners and controls. Front. Integr. Neurosci. 2018, 12, 26. [Google Scholar] [CrossRef] [PubMed]
- Gard, T.; Taquet, M.; Dixit, R.; Hölzel, B.K.; de Montjoye, Y.-A.; Brach, N.; Salat, D.H.; Dickerson, B.C.; Gray, J.R.; Lazar, S.W. Fluid intelligence and brain functional organization in aging yoga and meditation practitioners. Front. Aging Neurosci. 2014, 6, 76. [Google Scholar] [CrossRef] [PubMed]
- Ward, N.S. Chapter 11: Neurological Rehabilitation. In Functional Neuroimaging; Elsevier Inc.: Amsterdam, The Netherlands, 2013; Volume 110. [Google Scholar]
- Saxena, S.; Rauch, S.L. Functional neuroimaging and the neuroanatomy of obsessive-compulsive disorder. Psychiatr. Clin. N. Am. 2000, 23, 563–586. [Google Scholar] [CrossRef]
- Swain, J.E. Baby stimuli and the parent brain: Functional neuroimaging of the neural substrates of parent-infant attachment. Psychiatry (Edgmont) 2008, 5, 28. [Google Scholar] [PubMed]
- Bakker, M.; Verstappen, C.; Bloem, B.; Toni, I. Recent advances in functional neuroimaging of gait. J. Neural Transm. 2007, 114, 1323–1331. [Google Scholar] [CrossRef] [Green Version]
- Nowrangi, M.A.; Lyketsos, C.; Rao, V.; Munro, C.A. Systematic review of neuroimaging correlates of executive functioning: Converging evidence from different clinical populations. J. Neuropsychiatry Clin. Neurosci. 2014, 26, 114–125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sexton, C.E.; Betts, J.F.; Demnitz, N.; Dawes, H.; Ebmeier, K.P.; Johansen-Berg, H. A systematic review of MRI studies examining the relationship between physical fitness and activity and the white matter of the ageing brain. Neuroimage 2016, 131, 81–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimada, H.; Ishii, K.; Makizako, H.; Ishiwata, K.; Oda, K.; Suzukawa, M. Effects of exercise on brain activity during walking in older adults: A randomized controlled trial. J. Neuroeng. Rehabil. 2017, 14, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tabelow, K.; Polzehl, J. Statistical parametric maps for functional MRI experiments in R: The package fmri. J. Stat. Softw. 2011, 44, 6355. [Google Scholar] [CrossRef] [Green Version]
- Alač, M.; Hutchins, E. I see what you are saying: Action as cognition in fMRI brain mapping practice. J. Cogn. Cult. 2004, 4, 629–661. [Google Scholar] [CrossRef] [Green Version]
- Wager, T.D.; Atlas, L.Y.; Lindquist, M.A.; Roy, M.; Woo, C.-W.; Kross, E. An fMRI-based neurologic signature of physical pain. N. Engl. J. Med. 2013, 368, 1388–1397. [Google Scholar] [CrossRef] [Green Version]
- Fontes, E.B.; Okano, A.H.; De Guio, F.; Schabort, E.J.; Min, L.L.; Basset, F.A.; Stein, D.J.; Noakes, T.D. Brain activity and perceived exertion during cycling exercise: An fMRI study. Br. J. Sports Med. 2015, 49, 556–560. [Google Scholar] [CrossRef]
- Yu, Q.; Herold, F.; Becker, B.; Klugah-Brown, B.; Zhang, Y.; Perrey, S.; Veronese, N.; Müller, N.G.; Kramer, A.F.; Zou, L. Cognitive benefits of exercise interventions: An fMRI activation likelihood estimation meta-analysis. Brain Struct. Funct. 2021, 226, 601–619. [Google Scholar] [CrossRef]
- Ishihara, T.; Miyazaki, A.; Tanaka, H.; Matsuda, T. Identification of the brain networks that contribute to the interaction between physical function and working memory: An fMRI investigation with over 1,000 healthy adults. NeuroImage 2020, 221, 117152. [Google Scholar] [CrossRef]
- Misaki, M.; Kerr, K.L.; Ratliff, E.L.; Cosgrove, K.T.; Simmons, W.K.; Morris, A.S.; Bodurka, J. Beyond synchrony: The capacity of fMRI hyperscanning for the study of human social interaction. Soc. Cogn. Affect. Neurosci. 2021, 16, 84–92. [Google Scholar] [CrossRef]
- Pinti, P.; Tachtsidis, I.; Hamilton, A.; Hirsch, J.; Aichelburg, C.; Gilbert, S.; Burgess, P.W. The present and future use of functional near-infrared spectroscopy (fNIRS) for cognitive neuroscience. Ann. N. Y. Acad. Sci. 2020, 1464, 5. [Google Scholar] [CrossRef] [PubMed]
- Herold, F.; Wiegel, P.; Scholkmann, F.; Müller, N.G. Applications of functional near-infrared spectroscopy (fNIRS) neuroimaging in exercise–cognition science: A systematic, methodology-focused review. J. Clin. Med. 2018, 7, 466. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Lu, Y.; Zhou, C.; Wang, X. The effects of aerobic exercise on working memory in methamphetamine-dependent patients: Evidence from combined fNIRS and ERP. Psychol. Sport Exerc. 2020, 49, 101685. [Google Scholar] [CrossRef]
- Pelicioni, P.H.; Tijsma, M.; Lord, S.R.; Menant, J. Prefrontal cortical activation measured by fNIRS during walking: Effects of age, disease and secondary task. PeerJ 2019, 7, e6833. [Google Scholar] [CrossRef] [Green Version]
- Schack, J.; Pripp, A.H.; Mirtaheri, P.; Steen, H.; Güler, E.; Gjøvaag, T. Increased prefrontal cortical activation during challenging walking conditions in persons with lower limb amputation–an fNIRS observational study. Physiother. Theory Pract. 2020, 5, 1–11. [Google Scholar] [CrossRef]
- Xie, H.; Zhang, M.; Huo, C.; Xu, G.; Li, Z.; Fan, Y. Tai Chi Chuan exercise related change in brain function as assessed by functional near–infrared spectroscopy. Sci. Rep. 2019, 9, 13198. [Google Scholar] [CrossRef]
- Chen, W.L.; Wagner, J.; Heugel, N.; Suagr, J.; Lee, Y.W.; Conant, L.; Malloy, M.; Heffernan, J.; Quirk, B.; Zinos, A.; et al. Functional Near-Infrared Spectroscopy and Its Clinical Application in the Field of Neuroscience: Advances and Future Directions. Front. Neurosci. 2020, 14, 724. [Google Scholar] [CrossRef]
- Wang, C.-H.; Moreau, D.; Kao, S.-C. From the lab to the field: Potential applications of dry EEG systems to understand the brain-behavior relationship in sports. Front. Neurosci. 2019, 13, 893. [Google Scholar] [CrossRef] [Green Version]
- John, A.; Schöllhorn, W.I. Acute effects of instructed and self-created variable rope skipping on EEG brain activity and heart rate variability. Front. Behav. Neurosci. 2018, 12, 311. [Google Scholar] [CrossRef]
- Engchuan, P.; Wongsuphasawat, K.; Sittiprapaporn, P. Changes of EEG Power Spectra in Bench Press Weight Training Exercise. In Proceedings of the 14th International Conference on Electrical Engineering/Electronics, Computer, Telecommunications and Information Technology (ECTI-CON), Phuket, Thailand, 27–30 June 2017; pp. 13–16. [Google Scholar]
- Hong, S.-G.; Kim, J.-H.; Jun, T.-W. Effects of 12-week resistance exercise on electroencephalogram patterns and cognitive function in the elderly with mild cognitive impairment: A randomized controlled trial. Clin. J. Sport Med. 2018, 28, 500–508. [Google Scholar] [CrossRef]
- Gaur, S.; Panjwani, U.; Kumar, B. EEG Brain Wave Dynamics: A Systematic Review and Meta Analysis on Eff ect of Yoga on Mind Relaxation. J. Biomed. Res. Environ. Sci. 2020, 1, 353–362. [Google Scholar] [CrossRef]
- Nara, S.; Sheoran, P. Advancements in EEG source localisation methods. Int. J. Med. Eng. Inform. 2018, 10, 30–48. [Google Scholar] [CrossRef]







| Brain Region | Side | x | y | z | Voxels | t-Value |
|---|---|---|---|---|---|---|
| Precuneus (BA 31) | R | 11 | −74 | 23 | 52 | 8.52 |
| Anterior insula (BA 13) | L | −42 | −6 | −0 | 136 | 7.88 |
| Anterior cingulate cortex (BA 32) | R | 2 | 32 | 6 | 35 | 6.32 |
| Inferior frontal gyrus (BA 9) | L | −45 | 11 | 33 | 48 | 6.75 |
| Inferior parietal lobule (BA 3) | L | −45 | −24 | 44 | 75 | 7.50 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Srinivas, N.S.; Vimalan, V.; Padmanabhan, P.; Gulyás, B. An Overview on Cognitive Function Enhancement through Physical Exercises. Brain Sci. 2021, 11, 1289. https://doi.org/10.3390/brainsci11101289
Srinivas NS, Vimalan V, Padmanabhan P, Gulyás B. An Overview on Cognitive Function Enhancement through Physical Exercises. Brain Sciences. 2021; 11(10):1289. https://doi.org/10.3390/brainsci11101289
Chicago/Turabian StyleSrinivas, Narayanasamy Sai, Vijayaragavan Vimalan, Parasuraman Padmanabhan, and Balázs Gulyás. 2021. "An Overview on Cognitive Function Enhancement through Physical Exercises" Brain Sciences 11, no. 10: 1289. https://doi.org/10.3390/brainsci11101289