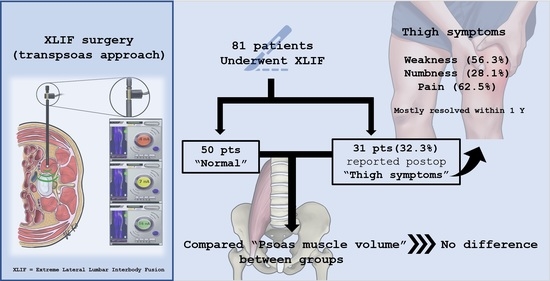
Psoas Major Muscle Volume Does Not Affect the Postoperative Thigh Symptoms in XLIF Surgery
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Smith, W.D.; Christian, G.; Serrano, S.; Malone, K.T. A comparison of perioperative charges and outcome between open and mini-open approaches for anterior lumbar discectomy and fusion. J. Clin. Neurosci. 2012, 19, 673–680. [Google Scholar] [CrossRef]
- Lucio, J.C.; Rodgers, J.A.; Vanconia, R.B.; Deluzio, K.J.; A Lehmen, J.; Rodgers, W. Economics of less invasive spinal surgery: An analysis of hospital cost differences between open and minimally invasive instrumented spinal fusion procedures during the perioperative period. Risk Manag. Healthc. Policy 2012, 5, 65–74. [Google Scholar] [PubMed] [Green Version]
- Rodgers, W.B.; Cox, C.S.; Gerber, E.J. Early Complications of Extreme Lateral Interbody Fusion in the Obese. J. Spinal Disord. Tech. 2010, 23, 393–397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ozgur, B.M.; Aryan, H.E.; Pimenta, L.; Taylor, W.R. Extreme Lateral Interbody Fusion (XLIF): A novel surgical technique for anterior lumbar interbody fusion. Spine J. 2006, 6, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Svennigsen, Å.F.; Dahlin, L.B. Repair of the Peripheral Nerve—Remyelination that Works. Brain Sci. 2013, 3, 1182–1197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kocot-Kępska, M.; Zajączkowska, R.; Mika, J.; Wordliczek, J.; Dobrogowski, J.; Przeklasa-Muszyńska, A. Peripheral Mechanisms of Neuropathic Pain—the Role of Neuronal and Non-Neuronal Interactions and Their Implications for Topical Treatment of Neuropathic Pain. Pharmaceuticals 2021, 14, 77. [Google Scholar] [CrossRef] [PubMed]
- Park, D.K.; Lee, M.J.; Lin, E.L.; Singh, K.; An, H.S.; Phillips, F.M. The relationship of intrapsoas nerves during a transpsoas approach to the lumbar spine: Anatomic study. J. Spinal Disord. Tech. 2010, 23, 223–228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benglis, D.M.; Vanni, S.; Levi, A.D. An anatomical study of the lumbosacral plexus as related to the minimally invasive transpsoas approach to the lumbar spine. J. Neurosurg. Spine 2009, 10, 139–144. [Google Scholar] [CrossRef]
- Moro, T.; Kikuchi, S.-I.; Konno, S.-I.; Yaginuma, H. An Anatomic Study of the Lumbar Plexus with Respect to Retroperitoneal Endoscopic Surgery. Spine 2003, 28, 423–427. [Google Scholar] [CrossRef] [PubMed]
- Cummock, M.D.; Vanni, S.; Levi, A.D.; Yu, Y.; Wang, M.Y. An analysis of postoperative thigh symptoms after minimally invasive transpsoas lumbar interbody fusion. J. Neurosurg. Spine 2011, 15, 11–18. [Google Scholar] [CrossRef]
- Gammal, I.D.; Spivak, J.M.; Bendo, J.A. Systematic Review of Thigh Symptoms after Lateral Transpsoas Interbody Fusion for Adult Patients with Degenerative Lumbar Spine Disease. Int. J. Spine Surg. 2015, 9, 62. [Google Scholar] [CrossRef] [Green Version]
- Tohmeh, A.G.; Rodgers, W.B.; Peterson, M.D. Dynamically evoked, discrete-threshold electromyography in the extreme lateral interbody fusion approach. J. Neurosurg. Spine 2011, 14, 31–37. [Google Scholar] [CrossRef]
- Di Stefano, G.; Di Lionardo, A.; Di Pietro, G.; Truini, A. Neuropathic Pain Related to Peripheral Neuropathies According to the IASP Grading System Criteria. Brain Sci. 2020, 11, 1. [Google Scholar] [CrossRef] [PubMed]
- Yam, M.F.; Loh, Y.C.; Tan, C.S.; Adam, S.K.; Manan, N.A.; Basir, R. General Pathways of Pain Sensation and the Major Neurotransmitters Involved in Pain Regulation. Int. J. Mol. Sci. 2018, 19, 2164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gordon, T. Peripheral Nerve Regeneration and Muscle Reinnervation. Int. J. Mol. Sci. 2020, 21, 8652. [Google Scholar] [CrossRef] [PubMed]
- Buric, J. Relationship between psoas muscle dimensions and post operative thigh pain. A possible preoperative evaluation factor. Int. J. Spine Surg. 2015, 9, 27. [Google Scholar] [CrossRef]
- Louie, P.K.; Narain, A.S.; Hijji, F.Y.; Yacob, A.; Yom, K.H.; Phillips, F.M.; Singh, K. Radiographic Analysis of Psoas Morphology and its Association With Neurovascular Structures at L4-5 With Reference to Lateral Approaches. Spine 2017, 42, E1386–E1392. [Google Scholar] [CrossRef]
- Pumberger, M.; Hughes, A.P.; Huang, R.R.; Sama, A.A.; Cammisa, F.P.; Girardi, F.P. Neurologic deficit following lateral lumbar interbody fusion. Eur. Spine J. 2011, 21, 1192–1199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tormenti, M.J.; Maserati, M.B.; Bonfield, C.M.; Okonkwo, D.O.; Kanter, A.S. Complications and radiographic correction in adult scoliosis following combined transpsoas extreme lateral interbody fusion and posterior pedicle screw instrumentation. Neurosurg. Focus 2010, 28, E7. [Google Scholar] [CrossRef]
- Rodgers, W.B.; Gerber, E.J.; Patterson, J. Intraoperative and early postoperative complications in extreme lateral interbody fusion: An analysis of 600 cases. Spine 2011, 36, 26–32. [Google Scholar] [CrossRef] [Green Version]
- Zeng, Z.Y.; Xu, Z.-W.; He, D.-W.; Zhao, X.; Ma, W.-H.; Ni, W.-F.; Song, Y.-X.; Zhang, J.-Q.; Yu, W.; Fang, X.-Q.; et al. Complications and Prevention Strategies of Oblique Lateral Interbody Fusion Technique. Orthop. Surg. 2018, 10, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.T.; Lee, H.J. Prone Position-Related Meralgia Paresthetica after Lumbar Spinal Surgery: A Case Report and Review of the Literature. J. Korean Neurosurg. Soc. 2008, 44, 392–395. [Google Scholar] [CrossRef] [PubMed]
- Menezes-Reis, R.; Bonugli, G.P.; Salmon, C.E.G.; Mazoroski, D.; Herrero, C.F.P.D.S.; Nogueira-Barbosa, M.H. Relationship of spinal alignment with muscular volume and fat infiltration of lumbar trunk muscles. PLoS ONE 2018, 13, e0200198. [Google Scholar] [CrossRef] [PubMed]



| N (%) | |
|---|---|
| Female | 38 (46.9) |
| History of previous lumbar surgery | 24 (29.6) |
| Diagnosis | |
| - ASD | 27 (33.3) |
| - Spondylolisthesis | 23 (28.4) |
| - DDD | 14 (17.3) |
| - Degenerative scoliosis | 11 (13.6) |
| - Spinal canal stenosis | 4 (4.9) |
| Operation | |
| - XLP | 24 (29.6) |
| - PPS | 48 (59.3) |
| - Alone | 9 (11.1) |
| Symptomatic Patients (n = 30) | Asymptomatic Patients (n = 51) | p-Value | |
|---|---|---|---|
| Mean age (year) | 66 | 67.6 | 0.138 |
| L1–2 | 2 (3.3%) | 4 (7.8%) | 0.454 |
| L2–3 | 14 (23.0%) | 7 (13.7%) | 0.138 |
| L3–4 | 1 (1.6%) | 12 (23.5%) | 0.155 |
| L4–5 | 13 (21.3%) | 18 (35.3%) | 1.000 |
| L3–5 | 0 (0%) | 7 (13.7%) | 1.000 |
| L2–5 | 0 (0%) | 3 (5.9%) | 1.000 |
| Thigh Symptoms | Symptomatic Patients | |
|---|---|---|
| Post-Op Day 1 | Post-Op 12 Months | |
| Pain | 20 (32.8%) | 2 (3.3%) |
| Numbness | 9 (14.8%) | 0 (0%) |
| Weakness | 18 (29.5%) | 1 (1.6%) |
| Patient | Level | Symptoms | ||
|---|---|---|---|---|
| Pain | Numbness | Weakness | ||
| 1 | L1–2 | + | + | + |
| Resolution (months) | No | 12 | 3 | |
| 2 | L2–3 | + | + | + |
| Resolution (months) | 6 | 3 | 3 | |
| 3 | L2–3 | + | + | |
| Resolution (months) | 3 | 1 | ||
| 4 | L2–3 | + | ||
| Resolution (months) | 1 | |||
| 5 | L2–3 | + | + | |
| Resolution (months) | No | 3 | ||
| 6 | L2–3 | + | ||
| Resolution (months) | 1 | |||
| 7 | L2–3 | + | ||
| Resolution (months) | 1 | |||
| 8 | L2–3 | + | + | |
| Resolution (months) | 6 | No | ||
| 9 | L2–3 | + | + | |
| Resolution (months) | 3 | 1 | ||
| 10 | L3–4 | + | ||
| Resolution (months) | 3 | |||
| 11 | L2–3 | + | ||
| Resolution (months) | 1 | |||
| 12 | L4–5 | + | + | + |
| Resolution (months) | 3 | 6 | 1 | |
| 13 | L2–3 | + | ||
| Resolution (months) | 3 | |||
| 14 | L4–5 | + | ||
| Resolution (months) | 3 | |||
| 15 | L4–5 | + | + | |
| Resolution (months) | 12 | 6 | ||
| 16 | L4–5 | + | ||
| Resolution (months) | 1 | |||
| 17 | L3–4 | + | + | |
| Resolution (months) | 3 | 1 | ||
| 18 | L2–3 | + | ||
| Resolution (months) | 1 | |||
| 19 | L4–5 | + | ||
| Resolution (months) | 12 | |||
| 20 | L4–5 | + | + | |
| Resolution (months) | 3 | 1 | ||
| 21 | L2–3 | + | ||
| Resolution (months) | 1 | |||
| 22 | L3–4 | + | ||
| Resolution (months) | 3 | |||
| 23 | L4–5 | + | + | |
| Resolution (months) | 6 | 1 | ||
| 24 | L2–3 | + | ||
| Resolution (months) | 3 | |||
| 25 | L2–3 | + | ||
| Resolution (months) | 3 | |||
| 26 | L4–5 | + | ||
| Resolution (months) | 6 | |||
| 27 | L2–3 | + | ||
| Resolution (months) | 1 | |||
| 28 | L4–5 | + | ||
| Resolution (months) | 6 | |||
| 29 | L4–5 | + | ||
| Resolution (months) | 6 | |||
| 30 | L4–5 | + | ||
| Resolution (months) | 3 | |||
| 31 | L4–5 | + | + | |
| Resolution (months) | 6 | 1 | ||
| 32 | L4–5 | + | ||
| Resolution (months) | 1 | |||
| n | Psoas Major Muscle Volume (cm3) | p Value | ||
|---|---|---|---|---|
| Mean ± SD | Median (Min, Max) | |||
| Pain | ||||
| No | 62 | 411.48 ± 113.95 | 420.30 (364.22, 531.31) | 0.895 |
| Yes | 19 | 419.04 ± 84.32. | 407.64 (365.93, 549.05) | |
| Numbness | ||||
| No | 72 | 421.76 ± 108.80 | 420.30 (324.72, 514.16) | 0.469 |
| Yes | 9 | 397.96 ± 118.41 | 407.64 (387.13, 552.46) | |
| Weakness | ||||
| No | 63 | 411.40 ± 115.87 | 424.87 (324.72, 514.16) | 0.887 |
| Yes | 18 | 419.67 ± 96.72 | 389.31 (351.30, 549.05) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yingsakmongkol, W.; Wathanavasin, W.; Jitpakdee, K.; Singhatanadgige, W.; Limthongkul, W.; Kotheeranurak, V. Psoas Major Muscle Volume Does Not Affect the Postoperative Thigh Symptoms in XLIF Surgery. Brain Sci. 2021, 11, 357. https://doi.org/10.3390/brainsci11030357
Yingsakmongkol W, Wathanavasin W, Jitpakdee K, Singhatanadgige W, Limthongkul W, Kotheeranurak V. Psoas Major Muscle Volume Does Not Affect the Postoperative Thigh Symptoms in XLIF Surgery. Brain Sciences. 2021; 11(3):357. https://doi.org/10.3390/brainsci11030357
Chicago/Turabian StyleYingsakmongkol, Wicharn, Waranyoo Wathanavasin, Khanathip Jitpakdee, Weerasak Singhatanadgige, Worawat Limthongkul, and Vit Kotheeranurak. 2021. "Psoas Major Muscle Volume Does Not Affect the Postoperative Thigh Symptoms in XLIF Surgery" Brain Sciences 11, no. 3: 357. https://doi.org/10.3390/brainsci11030357