1. Introduction
Increasing cadence in locomotion might be beneficial for a variety of reasons and auditory pacing could well be used to achieve this. For instance, with respect to walking it has been suggested that increasing cadence of stroke patients, who often have a reduced ability to modulate cadence, could help increase their walking speed and improve mobility [
1,
2]. Most stroke patients are able to adjust their cadence to an external beat [
3,
4,
5]. With regard to running, research has suggested that running injuries due to overload might be reduced by increasing cadence at a given speed [
6,
7]. Small increases in cadence (+ 10%, and corresponding decreases in step length [
8]) result in a reduction of energy absorption in the joints of the lower extremities [
7] as well as a decrease in braking impulse and instantaneous vertical loading rate [
9]. Runners are also able to couple their cadence to an external beat [
10,
11].
Auditory pacing is commonly used to prescribe a particular movement frequency in cyclic tasks, including locomotion. In locomotion, auditory pacing can be readily provided via a headset. Auditory cues in the form of a beat can be used to influence the movement pattern through a process known as auditory-motor synchronization [
11,
12,
13,
14,
15,
16,
17], defined here as the coordination of rhythmic movements, such as steps, to external stimuli, such as metronome beeps. Stable coordination between steps and cues is associated with a strong effect of pacing on the movement pattern [
4,
18]. The stability of auditory-motor coordination is often examined in terms of the variability of the relative phase between steps and cues, with lower variability representing higher stability [
4,
13,
19]. In addition, step adjustments to rhythm perturbations in the pacing signal can be used to assess the stability of auditory-motor coordination, with faster corrections representing higher stability [
4,
12,
20,
21].
Two factors influence auditory-motor coordination: coupling strength and frequency detuning [
22]. Coupling strength refers to how weak or strong the interaction is between two oscillators, here between rhythmic limb movements and auditory cues. Different types of pacing (e.g., one or two cues per movement cycle) are associated with different coupling strengths, which affect the stability of auditory-motor coordination [
22]. Most research on auditory pacing has been conducted in the context of finger and hand movements [
12,
23,
24,
25], but the same concept holds for auditory-motor coordination during locomotion [
13,
14,
26]. In finger and hand movement tasks, coupling is stronger for one cue per tap (1:1 ratio) than for one cue every other tap (1:2 ratio) [
22], resulting in superior auditory-motor coordination. One study compared a 1:1 ratio (pacing steps) to a 1:2 ratio (pacing strides) in walking [
4]. As for tapping, a superior auditory-motor coordination for the 1:1 ratio (one cue per step; stronger coupling) was found compared to the 1:2 ratio (one cue per stride, that is, one cue every other step; weaker coupling). In the present study, we sought to verify these coupling-strength effects for walking and to extend them to running using a within-subjects design requiring experienced runners (i.e., participants who could perform all conditions).
In tapping, synchronization between taps and cues typically occurs within a certain frequency range [
12,
27]. That is, if the difference between the preferred unpaced tapping frequency and the imposed pacing frequency becomes too large, synchronization becomes impossible. This relates to the second factor affecting auditory-motor coordination, frequency detuning, defined for two coupled oscillators as the mismatch between their intrinsic frequencies [
22,
28]. Modulating cadence with auditory pacing by definition implies detuning, as pacing frequencies differ from one’s preferred cadence. With a frequency mismatch, synchronization between steps and cues may or may not occur, depending on the strength of the coupling (i.e., weaker for pacing strides than for pacing steps) and the magnitude of the mismatch (i.e., how much the paced frequency differs from one’s preferred cadence in the absence of pacing). Hence, fewer occurrences of synchronization are to be expected for stride-based pacing (weaker coupling) than for step-based pacing (stronger coupling), and particularly so for pacing frequencies other than one’s preferred frequency. If synchronization does occur, detuning is expected to affect the stability of auditory-motor coordination [
13,
22], with superior auditory-motor coordination (i.e., lower relative-phase variability, faster responses to rhythm perturbations) at one’s preferred cadence (i.e., no detuning). Detuning also affects lead-lag relationships between steps and pacing cues during synchronized auditory-motor coordination [
12,
22]. Footfalls typically precede pacing cues (i.e., anticipation tendency [
12,
24]), with the magnitude of this phase lead depending on the frequency mismatch between the pacing frequency and one’s preferred cadence in the absence of pacing [
12,
13]. In line with this detuning effect, the intrinsically faster oscillator (i.e., walker/runner paced at slower-than-preferred cadence) tends to lead and the intrinsically slower oscillator (i.e., walker/runner paced at faster-than-preferred cadence) tends to lag the anticipation tendency seen in the absence of detuning (i.e., walker/runner paced at their preferred cadence) [
12,
13]. The mean relative phase between cues and steps indicates the phase lead/lag.
Not only do indicators of synchronization and the stability of auditory-motor coordination matter when one wants to modulate cadence with auditory pacing, but also the extent to which the prescribed cadence can be internalized when pacing is turned off. This modulating effect of pacing in terms of internalization of the prescribed cadence can be examined using a synchronization-continuation paradigm, with lower deviations from the prescribed cadence after removal of the pacing signal representing stronger internalization [
23,
29,
30,
31]. Furthermore, besides objective findings, the subjective experience of the user about the various pacing types matter for practical reasons such as compliance [
32]. Ideally, objective synchronization, stability and internalization findings match participants’ subjective experiences with the various types of cues.
In sum, the purpose of the present study was to compare the effectiveness of step-based and stride-based pacing for modulating walking and running cadences. We expected more synchronization and more stable auditory-motor coordination with step-based pacing (stronger coupling) for both walking and running, reflecting a superior cadence-modulating effect [
4,
18]. As a reflection of this, we expected participants’ subjective experiences about the various types of cues to match objective synchronization, auditory-motor coordination and internalization findings in that participants would best rate the pacing type with the strongest coupling (i.e., step-based pacing). Furthermore, we expected more stable auditory-motor coordination with congruent pacing (i.e., pacing matching one’s preferred cadence), and decreasing phase leads with faster pacing. Finally, we expected a larger detuning-related deviation from the prescribed cadence after removal of the pacing signal in slow and fast pacing conditions, with a change of cadence towards one’s preferred cadence.
3. Results
The comfortable speeds for walking and running were 5.22 ± 0.48 km/h and 10.47 ± 0.94 km/h, respectively. The corresponding preferred cadence for walking was 113.41 ± 5.95 steps/min (114.21 ± 6.27 steps/min after completing the measurements; ICC = 0.79; t(15) = −0.79, p = 0.44). For running, the preferred cadence was 162.20 ± 8.78 steps/min (164.18 ± 9.99 steps/min after completing the measurements; ICC = 0.78; t(15) = −1.44, p = 0.17).
3.1. Synchronization Phase
The number of participants who achieved synchronization differed significantly across conditions (
Χ2(16) = 31.97,
p = 0.001). Significantly less participants achieved synchronization in the running conditions than in the walking conditions (
T = 0,
r = −0.68;
Table 2). Furthermore, synchronization was achieved less often for the slow pacing frequency compared to preferred (
T = 0,
r = −0.64) and fast (
T = 6,
r = −0.50) pacing frequencies (
Table 2). Five out of 16 participants achieved synchronization in all conditions, and three out of 16 participants achieved synchronization in (less than) half of the conditions. The other eight participants achieved synchronization in 9 to 11 out of 12 conditions. The participants who synchronized with the pacing signal did so with the normalized cadences shown in
Table 2.
Due to the lack of synchronization in at least one condition, 11 participants would be excluded from the planned repeated-measures ANOVA. Given the distribution of invalid conditions in the running conditions with detuning, we instead used a 2 (pacing type) × 2 (locomotion) repeated-measures ANOVA including only the conditions with the preferred pacing frequency, and a 2 (pacing type) × 3 (pacing frequency) repeated-measures ANOVA including only the walking conditions. This led to the inclusion of 13 and 11 participants, respectively.
The constant error of the normalized cadence during synchronization with preferred pacing frequency was 0.001 ± 0.001. There were no significant differences in constant error across locomotion types and pacing types. However, there was a significant effect of frequency on the constant error, F(1.20, 11.97) = 56.37, p = 0.000, ηp2 = 0.85 (Greenhouse-Geisser corrected values are reported, because Mauchly’s test was significant, p = 0.007). Post-hoc tests with Bonferroni correction revealed a small, but significantly higher constant error for the slow (0.009 ± 0.005) and fast (0.012 ± 0.001) pacing frequencies compared to the preferred pacing frequency (0.001 ± 0.001; p < 0.001).
For the walking conditions, pacing frequency had a significant effect on the mean relative phase,
F(1.24, 12.37) = 18.56,
p = 0.001,
ηp2 = 0.65. Post-hoc tests with Bonferroni correction revealed that the mean relative phase of slow (38.83° ± 22.27°), preferred (18.93° ± 10.85°), and fast (−0.79° ± 13.09°) pacing-frequency conditions all differed significantly from each other (
p < 0.05;
Figure 3). For the conditions with the preferred pacing frequency, a trend suggested a larger mean relative phase for running (33.81° ± 21.61°) than for walking (21.66° ± 11.18°),
p = 0.053. No significant main effect of pacing type was found, nor any significant interactions.
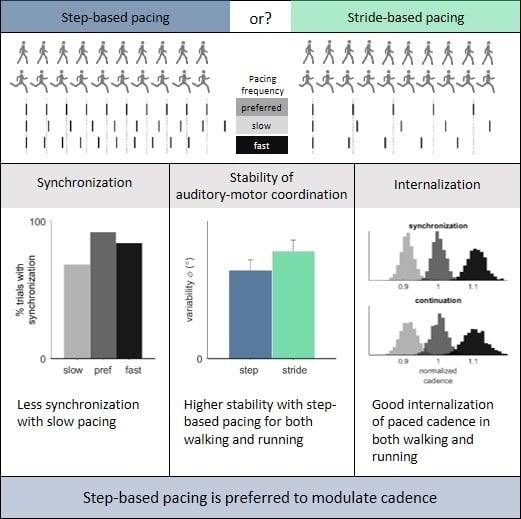
Variability of the relative phase was significantly higher with stride-based (5.48° ± 2.01°) than step-based pacing (4.50° ± 1.92°),
F(1, 12) = 8.68,
p = 0.012,
ηp2 = 0.42 (
Figure 4a). There was a significant effect of frequency on the variability of the relative phase in walking (
F(2, 20) = 3.75,
p = 0.042,
ηp2 = 0.27). Post-hoc tests with Bonferroni correction revealed no significant individual differences between slow (5.72° ± 0.55°), preferred (4.78° ± 0.41°), and fast (5.75° ± 0.58°) pacing frequencies (
Figure 4b). No significant effect of locomotion was found, nor any significant interactions.
3.2. Perturbation Analysis
How often the different responses to the perturbations occurred varied across conditions (
Figure 5 and
Figure 6). Friedman’s ANOVA indicated that there were significant differences in the number of typical responses (|60°|) across conditions (
Χ2(11) = 63.95,
p < 0.001;
Figure 5). Wilcoxon tests with Bonferroni correction were used to make specific comparisons. Participants had less typical responses for running (3.51 ± 1.98; out of 8 perturbations) than for walking (5.22 ± 2.11;
T = 0,
r = 0.88), and less typical responses for slow pacing frequency conditions (3.17 ± 2.11) than for preferred frequency conditions (5.55 ± 1.95;
T = 1.5,
r = 0.86). The number of typical responses did not differ between step-based (4.36 ± 1.96) and stride-based (4.36 ± 2.15) pacing conditions. Similar but opposite differences were found for the number of invalid responses (
Χ2(11) = 63.88,
p < 0.001;
T = 0,
r = 0.88;
T = 1.5,
r = 0.86, respectively;
Figure 5).
Qualitative inspection of
Figure 5 and
Figure 6 reveals that with detuning, atypical (|120°| and |300°|) responses occur more often and with step responses in the direction of the preferred cadence. That is, participants predominantly adopt a slower-step response to correct for a phase-advance perturbation (−60°) with fast pacing frequency (yielding −120° and −300° adjustments) and a faster-step response to correct a phase-delay perturbation (+60°) with slow pacing frequency (yielding +120° and +300° adjustments [
4]).
For typical responses, the number of steps needed to correct for a perturbation were compared with a 2 (pacing type) × 2 (locomotion) repeated-measures ANOVA (
N = 15; preferred frequency conditions only), and with a 2 (pacing type) × 3 (pacing frequency) repeated-measures ANOVA (
N = 12; walking conditions only). There were no significant main effects of pacing type, locomotion or the pacing type x locomotion interaction (
p > 0.05) on the number of steps needed to correct for a perturbation with the preferred pacing frequency. For the walking conditions, there was a significant effect of frequency on the number of steps needed to correct,
F(2, 22) = 8.06,
p = 0.002,
ηp2 = 0.42. Post-hoc tests showed that this number was significantly higher for slow (9.66 ± 1.84) than fast (7.81 ± 1.48) pacing frequencies (
p = 0.011), but neither of those differed significantly from the number of steps needed for the preferred pacing frequency (9.12 ± 1.77). Furthermore, there was a significant interaction of pacing type and pacing frequency,
F(2, 22) = 4.77,
p = 0.019,
ηp2 = 0.30 (
Figure 7). Post-hoc tests with Bonferroni correction revealed that for the preferred pacing frequency, the number of steps needed to correct for a perturbation was significantly lower with step-based (8.41 ± 1.96) than with stride-based (9.83 ± 1.80) pacing,
p = 0.003, in the absence of significant differences between pacing types for slow and fast pacing frequencies.
3.3. Continuation Analysis
If stable auditory-motor coordination was not achieved during the final 50 steps of the synchronization phase, participants were excluded from the analysis of the continuation phase.
Figure 8 shows the histograms of the normalized cadence data separately for the synchronization and continuation parts of the conditions for the nine participants who achieved synchronization in all walking trials. The figure shows that the cadence, by and large, remains within the correct range during continuation. The tables above the separate figures show the percentage of steps with the cadence in the corresponding range per condition.
The constant error between the target cadence and the performed cadences during continuation were compared with a 2 (pacing type) × 2 (locomotion) repeated-measures ANOVA (
N = 9; preferred frequency conditions only), and with a 2 (pacing type) × 3 (pacing frequency) repeated-measures ANOVA (
N = 9; walking conditions only). In line with the qualitative inspection of
Figure 8, no significant main or interaction effects of pacing type and locomotion were found in the preferred frequency conditions (0.002 ± 0.009). In walking, there was a significant effect of pacing frequency on the constant error,
F(2, 16) = 3.81,
p = 0.044,
ηp2 = 0.32. Post-hoc tests with Bonferroni correction revealed a higher constant error for the slow pacing frequency (0.019 ± 0.018) compared to the preferred frequency (0.001 ± 0.015,
p < 0.05).
Figure 8 indeed shows that the histograms for the slow pacing frequency are shifted somewhat to the right, that is, in the direction of one’s preferred cadence. No significant interactions were found.
3.4. Questionnaire
The results of the questionnaire are presented in
Figure 9. Individual participant ratings are available in the
Supplementary Materials. Participants rated their synchronization performance to be better for step-based (73.07 ± 17.00) than for stride-based pacing (59.37 ± 24.67;
F(1, 14) = 6.06,
p = 0.027,
ηp2 = 0.30). They further rated comfort higher for step-based (66.47 ± 18.71) than for stride-based (55.13 ± 22.73) pacing (
F(1, 14) = 4.97,
p = 0.043,
ηp2 = 0.26). Difficulty and enjoyment rates did not differ between step-based and stride-based pacing (
p > 0.05). No significant differences in the ratings between walking and running were found, nor any significant interactions.
4. Discussion
In this study, we compared, in sixteen experienced runners, the effectiveness of step-based pacing and stride-based pacing for modulating and internalizing cadence in walking and running, for pacing frequencies slower than, equal to, and faster than the preferred cadence of the participants. We expected synchronization to be achieved more often with step-based (stronger coupling) than with stride-based pacing. However, no evidence was found for this expectation. If synchronization was achieved, we further expected auditory-motor coordination to be more stable for step-based pacing than for stride-based pacing for both walking and running. Auditory-motor coordination was indeed more stable with step-based pacing, as evidenced by significantly lower variability in the relative phase between steps and cues. Furthermore, fewer steps were needed to correct for a perturbation with step-based pacing than stride-based pacing, but only with preferred pacing frequency in walking; no significant main effect of pacing type was found. These findings indicate that stronger coupling (i.e., pacing steps) leads to more stable auditory-motor coordination, as was found in a previous study reporting superior auditory-motor synchronization for step-based pacing in walking [
4]. With regard to the participants’ preference, ratings suggested that step-based pacing was perceived to be more comfortable for both walking and running and led to a higher perceived performance than stride-based pacing. These subjective ratings in favor of step-based pacing are consistent with the objective findings showing superior coordinative stability of step-based pacing. In view of the superior auditory-motor coordination and better subjective ratings by the participants, we would advise walkers and runners (and their therapists and trainers) to opt for step-based pacing rather than stride-based pacing for the purpose of cadence modulation.
In the present study, we also systematically manipulated detuning, and expected that participants would achieve synchronization more often with pacing matching one’s preferred frequency (no detuning) than with slower-than-preferred and faster-than-preferred pacing frequencies (detuning). More participants were excluded in conditions with detuning due to the absence of synchronization, and there were less typical responses to perturbations, but only for conditions with a slower-than-preferred pacing frequency. This suggests that synchronizing to a slower-than-preferred pacing signal is more difficult than synchronizing to a pacing signal that matches one’s preferred cadence, but synchronizing to a faster-than-preferred pacing frequency is not. If synchronization was achieved, we expected auditory-motor coordination to be most stable for the preferred pacing frequency conditions (no detuning). Coordination was indeed more stable (lower variability of the relative phase) without detuning, which is consistent with the literature [
13]. Furthermore, the correction after perturbations was slower with slow than fast pacing frequency, but neither was significantly different from the preferred frequency. These combined results suggest that the negative effects of detuning on the stability of the auditory-motor coordination are more pronounced for slow than for fast pacing. The goal of the present study was to modulate cadence with the practical application of reducing injury. The increase in cadence of 10% recommended for that purpose [
6,
7] seems to be feasible with auditory pacing, with little loss of stability of auditory-motor coordination and, as will be discussed below, with internalization of this faster cadence after a relatively brief period of pacing.
We expected a detuning-related change of the cadence in the direction of the preferred cadence. During the synchronization phase, the constant error was indeed positive for the slow pacing frequency, indicating that the cadence tended to be somewhat faster than the slow pacing frequency, but it was also positive with fast pacing. In addition, detuning did have the expected effect on the lead-lag relationship between steps and pacing cues, with a larger phase lead with slow and a smaller phase lead (in some cases a phase lag) for fast pacing [
13], as evidenced respectively by a significantly higher and lower mean relative phase compared to the preferred pacing frequency, confirming that the inherently faster oscillator tends to lead [
12,
13]. Furthermore, the participants who achieved synchronization did remain at the target cadence after the removal of the pacing signal. We expected the constant error during continuation to be larger (positive) for the slow and smaller (negative) for the fast pacing frequency than for the preferred pacing frequency, but constant error, albeit low, was only significantly larger for the slow compared to the preferred pacing frequency, indicating a slightly worse internalization of the slower-than-preferred target frequency; no significant difference in constant error between the fast and preferred pacing was found. Participants were able to continue at the target cadence for 100 steps after removal of the pacing signal, provided that footfalls were synchronized to the pacing signal in the synchronization phase, since unsynchronized participants were excluded from this analysis.
We did not expect synchronization and stability of auditory-motor coordination to be different for walking and running. However, there were more conditions in which synchronization was not achieved, and more invalid responses to perturbations for running compared to walking, suggesting that synchronization was more difficult to achieve for running, especially at slow pacing frequencies. A plausible explanation for this difference is not readily apparent, but it may be the case that running, in particular on a treadmill, is a less automated activity than walking, and therefore more susceptible when combined with another attention-demanding task like synchronizing footfalls to an auditory metronome. Previous studies have shown that paced walking is more attention demanding than unpaced walking [
38,
39], and the same probably holds for running, but perhaps in a stronger manner. Note, however, that if synchronization was achieved, auditory-motor coordination was not significantly different for walking and running.
As already intimated, our findings may have practical implications for applying auditory pacing for modulating and internalizing cadence in the fields of rehabilitation and sports. However, before generalizing the current results to practice, a couple of limitations should be taken into account. Firstly, three of the participants did not achieve synchronization in (more than) half of the conditions. This could indicate that these participants had difficulty with auditory-motor synchronization in general [
40]. For such poor synchronizers, auditory pacing will not be an effective method for modulating cadence. Furthermore, in this study participants walked and ran on a treadmill. Treadmill locomotion differs somewhat from over-ground locomotion since the speed must be kept constant to avoid falling off the treadmill, implying that participants needed to tightly control their speed in light of this constraint [
41]. Consequently, modulating cadence through pacing also implied modulating step lengths (e.g., an increase in cadence is accompanied by a decrease in step length to maintain the same speed). In over-ground locomotion, in contrast, speed and step length may be adjusted independently of one another when modulating cadence. Increasing cadence over-ground through pacing may thus result in increased step lengths (and hence speed), which may be problematic in that greater step lengths are associated with injury risk in running [
8].
Given the aforementioned limitations, our recommendations for future research are to use a poor ability to synchronize as an exclusion criterion, as the poor synchronizers were excluded from most analyses in this study. In addition, we recommend future studies designed to extend our findings to over-ground running to pace cadence in relation to the actual speed in over-ground running, that is, if the use of auditory pacing is intended for modulating cadence with the aim of reducing injury risk. In relation to the latter recommendation, it is important to note that the energetically optimal cadence is faster than the preferred cadence in both treadmill [
42,
43] and over-ground [
44] running, and can be determined as the cadence corresponding to the lowest heart rate for each speed [
42,
43,
44]. We thus recommend future research to modulate cadence towards this energetically optimal cadence, as it may reduce both energy cost and injury risk.