Perfusion Patterns in Patients with Chronic Limb-Threatening Ischemia versus Control Patients Using Near-Infrared Fluorescence Imaging with Indocyanine Green
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Patient Characteristics
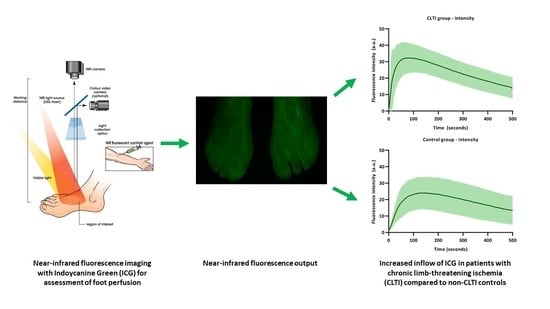
3.2. ICG NIR Fluorescence Parameters
3.3. Time–Intensity Curves
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Weitz, J.I.; Byrne, J.; Clagett, G.P.; Farkouh, M.E.; Porter, J.M.; Sackett, D.L.; Strandness, D.E., Jr.; Taylor, L.M. Diagnosis and treatment of chronic arterial insufficiency of the lower extremities: A critical review. Circulation 1996, 94, 3026–3049. [Google Scholar] [CrossRef] [PubMed]
- Aboyans, V.; Ricco, J.B.; Bartelink, M.E.L.; Bjorck, M.; Brodmann, M.; Cohnert, T.; Collet, J.P.; Czerny, M.; De Carlo, M.; Debusa, S.; et al. 2017 ESC Guidelines on the Diagnosis and Treatment of Peripheral Arterial Diseases, in collaboration with the European Society for Vascular Surgery (ESVS). Rev. Esp. Cardiol. 2018, 71, 111. [Google Scholar] [CrossRef] [PubMed]
- Krishna, S.M.; Moxon, J.V.; Golledge, J. A review of the pathophysiology and potential biomarkers for peripheral artery disease. Int. J. Mol. Sci. 2015, 16, 11294–11322. [Google Scholar] [CrossRef] [Green Version]
- Conte, M.S.; Bradbury, A.W.; Kolh, P.; White, J.V.; Dick, F.; Fitridge, R.; Mills, J.L.; Ricco, J.B.; Suresh, K.R.; Murad, M.H.; et al. Global vascular guidelines on the management of chronic limb-threatening ischemia. J. Vasc. Surg. 2019, 69, 3S–125S.e40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abularrage, C.J.; Sidawy, A.N.; Aidinian, G.; Singh, N.; Weiswasser, J.M.; Arora, S. Evaluation of the microcirculation in vascular disease. J. Vasc. Surg. 2005, 42, 574–581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Misra, S.; Shishehbor, M.H.; Takahashi, E.A.; Aronow, H.D.; Brewster, L.P.; Bunte, M.C.; Kim, E.S.H.; Lindner, J.R.; Rich, K.; American Heart Association Council on Peripheral Vascular Disease; et al. Perfusion Assessment in Critical Limb Ischemia: Principles for Understanding and the Development of Evidence and Evaluation of Devices: A Scientific Statement From the American Heart Association. Circulation 2019, 140, e657–e672. [Google Scholar] [CrossRef] [Green Version]
- van den Hoven, P.; Ooms, S.; van Manen, L.; van der Bogt, K.E.A.; van Schaik, J.; Hamming, J.F.; Vahrmeijer, A.L.; van der Vorst, J.R.; Mieog, J.S.D. A systematic review of the use of near-infrared fluorescence imaging in patients with peripheral artery disease. J. Vasc. Surg. 2019, 70, 286–297.e1. [Google Scholar] [CrossRef]
- Kikuchi, S.; Miyake, K.; Tada, Y.; Uchida, D.; Koya, A.; Saito, Y.; Ohura, T.; Azuma, N. Laser speckle flowgraphy can also be used to show dynamic changes in the blood flow of the skin of the foot after surgical revascularization. Vascular 2019, 27, 242–251. [Google Scholar] [CrossRef] [Green Version]
- Cindil, E.; Erbas, G.; Akkan, K.; Cerit, M.N.; Sendur, H.N.; Zor, M.H.; Ilgit, E. Dynamic Volume Perfusion CT of the Foot in Critical Limb Ischemia: Response to Percutaneous Revascularization. AJR Am. J. Roentgenol. 2020, 214, 1398–1408. [Google Scholar] [CrossRef]
- Driessen, C.; Arnardottir, T.H.; Lorenzo, A.R.; Mani, M.R. How should indocyanine green dye angiography be assessed to best predict mastectomy skin flap necrosis? A systematic review. J. Plast. Reconstr. Aesthet. Surg. 2020, 73, 1031–1042. [Google Scholar] [CrossRef]
- Dupree, A.; Riess, H.; Detter, C.; Debus, E.S.; Wipper, S.H. Utilization of indocynanine green fluorescent imaging (ICG-FI) for the assessment of microperfusion in vascular medicine. Innov. Surg. Sci. 2018, 3, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Vahrmeijer, A.L.; Hutteman, M.; van der Vorst, J.R.; van de Velde, C.J.; Frangioni, J.V. Image-guided cancer surgery using near-infrared fluorescence. Nat. Rev. Clin. Oncol. 2013, 10, 507–518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schaafsma, B.E.; Mieog, J.S.; Hutteman, M.; van der Vorst, J.R.; Kuppen, P.J.; Lowik, C.W.; Frangioni, J.V.; van de Velde, C.J.; Vahrmeijer, A.L. The clinical use of indocyanine green as a near-infrared fluorescent contrast agent for image-guided oncologic surgery. J. Surg. Oncol. 2011, 104, 323–332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parmeshwar, N.; Sultan, S.M.; Kim, E.A.; Piper, M.L. A Systematic Review of the Utility of Indocyanine Angiography in Autologous Breast Reconstruction. Ann. Plast. Surg. 2020, 85, 601–606. [Google Scholar] [CrossRef]
- Zimmermann, A.; Roenneberg, C.; Wendorff, H.; Holzbach, T.; Giunta, R.E.; Eckstein, H.H. Early postoperative detection of tissue necrosis in amputation stumps with indocyanine green fluorescence angiography. Vasc. Endovascular. Surg. 2010, 44, 269–273. [Google Scholar] [CrossRef] [PubMed]
- Terasaki, H.; Inoue, Y.; Sugano, N.; Jibiki, M.; Kudo, T.; Lepantalo, M.; Venermo, M. A quantitative method for evaluating local perfusion using indocyanine green fluorescence imaging. Ann. Vasc. Surg. 2013, 27, 1154–1161. [Google Scholar] [CrossRef]
- Kang, Y.; Lee, J.; Kwon, K.; Choi, C. Application of novel dynamic optical imaging for evaluation of peripheral tissue perfusion. Int. J. Cardiol. 2010, 145, e99–e101. [Google Scholar] [CrossRef]
- Igari, K.; Kudo, T.; Uchiyama, H.; Toyofuku, T.; Inoue, Y. Indocyanine green angiography for the diagnosis of peripheral arterial disease with isolated infrapopliteal lesions. Ann. Vasc. Surg. 2014, 28, 1479–1484. [Google Scholar] [CrossRef]
- Brouwers, J.; van Doorn, L.P.; van Wissen, R.C.; Putter, H.; Hamming, J.F. Using maximal systolic acceleration to diagnose and assess the severity of peripheral artery disease in a flow model study. J. Vasc. Surg. 2020, 71, 242–249. [Google Scholar] [CrossRef] [Green Version]
- Kang, Y.; Lee, J.; Kwon, K.; Choi, C. Dynamic fluorescence imaging of indocyanine green for reliable and sensitive diagnosis of peripheral vascular insufficiency. Microvasc. Res. 2010, 80, 552–555. [Google Scholar] [CrossRef]
- Zimmermann, A.; Roenneberg, C.; Reeps, C.; Wendorff, H.; Holzbach, T.; Eckstein, H.H. The determination of tissue perfusion and collateralization in peripheral arterial disease with indocyanine green fluorescence angiography. Clin. Hemorheol. Microcirc. 2012, 50, 157–166. [Google Scholar] [CrossRef]
- Igari, K.; Kudo, T.; Uchiyama, H.; Toyofuku, T.; Inoue, Y. Quantitative evaluation of microvascular dysfunction in peripheral neuropathy with diabetes by indocyanine green angiography. Diabetes Res. Clin. Pract. 2014, 104, 121–125. [Google Scholar] [CrossRef]
- Venermo, M.; Settembre, N.; Alback, A.; Vikatmaa, P.; Aho, P.S.; Lepantalo, M.; Inoue, Y.; Terasaki, H. Pilot Assessment of the Repeatability of Indocyanine Green Fluorescence Imaging and Correlation with Traditional Foot Perfusion Assessments. Eur. J. Vasc. Endovasc. Surg. 2016, 52, 527–533. [Google Scholar] [CrossRef] [Green Version]
- Nishizawa, M.; Igari, K.; Kudo, T.; Toyofuku, T.; Inoue, Y.; Uetake, H. A Comparison of the Regional Circulation in the Feet between Dialysis and Non-Dialysis Patients using Indocyanine Green Angiography. Scand. J. Surg. 2016, 106, 249–254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goncalves, L.N.; van den Hoven, P.; van Schaik, J.; Leeuwenburgh, L.; Hendricks, C.H.F.; Verduijn, P.S.; van der Bogt, K.E.A.; van Rijswijk, C.S.P.; Schepers, A.; Vahrmeijer, A.L.; et al. Perfusion Parameters in Near-Infrared Fluorescence Imaging with Indocyanine Green: A Systematic Review of the Literature. Life 2021, 11, 433. [Google Scholar] [CrossRef] [PubMed]
- Frangioni, J.V. In vivo near-infrared fluorescence imaging. Curr. Opin. Chem. Biol. 2003, 7, 626–634. [Google Scholar] [CrossRef] [PubMed]
- Rossi, M.; Carpi, A. Skin microcirculation in peripheral arterial obliterative disease. Biomed. Pharmacother. 2004, 58, 427–431. [Google Scholar] [CrossRef]
- Mendes-Pinto, D.; Rodrigues-Machado, M.D.G.; Navarro, T.P.; Dardik, A. Association Between Critical Limb Ischemia, the Society for Vascular Surgery Wound, Ischemia and Foot Infection (WIfI) Classification System and Arterial Stiffness. Ann. Vasc. Surg. 2020, 63, 250–258.e2. [Google Scholar] [CrossRef]
- Yu, S.; McEniery, C.M. Central Versus Peripheral Artery Stiffening and Cardiovascular Risk. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 1028–1033. [Google Scholar] [CrossRef]
- Pruimboom, T.; van Kuijk, S.M.J.; Qiu, S.S.; van den Bos, J.; Wieringa, F.P.; van der Hulst, R.; Schols, R.M. Optimizing Indocyanine Green Fluorescence Angiography in Reconstructive Flap Surgery: A Systematic Review and Ex Vivo Experiments. Surg. Innov. 2020, 27, 103–119. [Google Scholar] [CrossRef]
- Lutken, C.D.; Achiam, M.P.; Svendsen, M.B.; Boni, L.; Nerup, N. Optimizing quantitative fluorescence angiography for visceral perfusion assessment. Surg. Endosc. 2020, 34, 5223–5233. [Google Scholar] [CrossRef] [PubMed]




| CLTI | Controls | |
|---|---|---|
| N (limbs) | 19 (28) | 16 (32) |
| Age (SD) | 70.4 (7.5) | 66.6 (12.3) |
| Diabetes Mellitus (%) | 9 (47.4) | 3 (18.8) |
| Hypertension (%) | 15 (78.9) | 7 (43.8) |
| Active smoking (%) | 5 (26.3) | 1 (6.3) |
| Fontaine stage limbs, n (%) | ||
| 3 | 18 (64.3) | - |
| 4 | 10 (35.7) | - |
| Mean ABI (SD) | 0.77 (0.34) | 1.11 (0.10) |
| Mean TP (SD) | 44 (25) | 106 (22) |
| Acceleration (SD) | 0.93 (1.23) | - |
| Parameter | CLTI | Controls | p-Value |
|---|---|---|---|
| Maximum intensity (SD) | 37.9 (14.4) | 25.8 (10.8) | 0.000 |
| Maximum slope ingress (SD) | 2.0 (2.5) | 0.6 (0.4) | 0.000 |
| Normalized maximum slope (SD) | 4.2 (3.1) | 2.4 (1.2) | 0.000 |
| Ingress rate (SD) | 1.0 (1.7) | 0.2 (0.2) | 0.000 |
| AUC ingress 10 (SD) | 47.4 (2.2) | 48.8 (3.3) | 0.073 |
| AUC ingress (SD) | 71.4 (6.3) | 70.6 (3.8) | 0.213 |
| Tmax (SD) | 90.5 (53.4) | 143.3 (64.5) | 0.002 |
| Maximum slope egress (SD) | 0.5 (0.7) | 0.2 (0.1) | 0.005 |
| Normalized maximum slope egress (SD) | 1.0 (0.9) | 0.8 (0.3) | 0.733 |
| AUC egress 60 (SD) | 92.8 (10.0) | 96.7 (1.8) | 0.113 |
| AUC egress 120 (SD) | 87.9 (11.9) | 92.8 (2.3) | 0.127 |
| AUC egress 180 (SD) | 82.9 (12.9) | 88.3 (4.3) | 0.164 |
| AUC egress 240 (SD) | 78.2 (13.3) | 83.8 (5.4) | 0.168 |
| AUC egress 300 (SD) | 73.7 (13.5) | 73.3 (6.0) | 0.271 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Van Den Hoven, P.; Goncalves, L.N.; Quax, P.H.A.; Van Rijswijk, C.S.P.; Van Schaik, J.; Schepers, A.; Vahrmeijer, A.L.; Hamming, J.F.; Van Der Vorst, J.R. Perfusion Patterns in Patients with Chronic Limb-Threatening Ischemia versus Control Patients Using Near-Infrared Fluorescence Imaging with Indocyanine Green. Biomedicines 2021, 9, 1417. https://doi.org/10.3390/biomedicines9101417
Van Den Hoven P, Goncalves LN, Quax PHA, Van Rijswijk CSP, Van Schaik J, Schepers A, Vahrmeijer AL, Hamming JF, Van Der Vorst JR. Perfusion Patterns in Patients with Chronic Limb-Threatening Ischemia versus Control Patients Using Near-Infrared Fluorescence Imaging with Indocyanine Green. Biomedicines. 2021; 9(10):1417. https://doi.org/10.3390/biomedicines9101417
Chicago/Turabian StyleVan Den Hoven, Pim, Lauren N. Goncalves, Paulus H. A. Quax, Catharina S. P. Van Rijswijk, Jan Van Schaik, Abbey Schepers, Alexander L. Vahrmeijer, Jaap F. Hamming, and Joost R. Van Der Vorst. 2021. "Perfusion Patterns in Patients with Chronic Limb-Threatening Ischemia versus Control Patients Using Near-Infrared Fluorescence Imaging with Indocyanine Green" Biomedicines 9, no. 10: 1417. https://doi.org/10.3390/biomedicines9101417