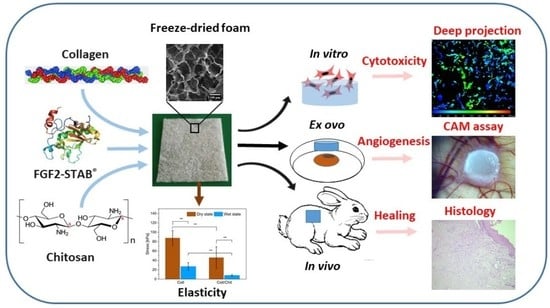
Healing and Angiogenic Properties of Collagen/Chitosan Scaffolds Enriched with Hyperstable FGF2-STAB® Protein: In Vitro, Ex Ovo and In Vivo Comprehensive Evaluation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Scaffold Preparation
2.2. Swelling Ratio
2.3. Hydrolytic Degradation
2.4. Enzymatic Degradation
2.5. Scaffold Morphology
2.6. Mechanical Testing
2.7. Cells and Culture Conditions
2.7.1. Cell Viability, Cell Adhesion and Proliferation on 3D Scaffolds
2.7.2. Cell Metabolic Activity Analysis by the MTS Test
2.7.3. Relative Collagen Type I mRNA Expression
2.8. Angiogenic Properties by Ex Ovo CAM Assay
2.9. In Vivo Evaluation in a Rabbit Model
2.9.1. Histological Assessment of Skin Formation
2.9.2. Gene Expression Analysis of Skin by RT-qPCR
2.10. Statistical Analysis
3. Results
3.1. The Effect of Chitosan on Scaffold Swelling Ability
3.2. The Effect of Chitosan on Hydrolytic Degradation
3.3. The Effect of Chitosan on Enzymatic Degradation
3.4. The Effect of Chitosan on the Scaffold Stiffness
3.5. The Effect of FGF2-STAB® on the Scaffold Structure
3.6. The Effect of FGF2-STAB® on Biological Properties
3.7. The Effect of FGF2-STAB® on Angiogenesis Evaluation
3.8. The Effect of FGF2-STAB® on In Vivo Biocompatibility
3.9. The Effect of FGF2-STAB® on Gene Expression by RT-qPCR
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yan, W.C.; Davoodi, P.; Vijayavenkataraman, S.; Tian, Y.; Ng, W.C.; Fuh, J.Y.H.; Robinson, K.S.; Wang, C.H. 3D bioprinting of skin tissue: From pre-processing to final product evaluation. Adv. Drug Deliv. Rev. 2018, 132, 270–295. [Google Scholar] [CrossRef]
- Mathes, S.H.; Ruffner, H. The use of skin models in drug development. Adv. Drug Deliv. Rev. 2014, 69–70, 81–102. [Google Scholar] [CrossRef]
- Parenteau, N.L.; Bilbo, P.; Nolte, C.J.M.; Mason, V.S.; Rosenberg, M. The organotypic culture of human skin keratinocytes and fibroblasts to achieve form and function. Cytotechnology 1992, 9, 163–171. [Google Scholar] [CrossRef]
- Shpichka, A.; Butnaru, D.; Bezrukov, E.A.; Sukhanov, R.B.; Atala, A.; Burdukovskii, V.; Zhang, Y.; Timashev, P. Skin tissue regeneration for burn injury. Stem Cell Res. Ther. 2019, 10, 94. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Ma, L.; Gao, C. Facile fabrication of the glutaraldehyde cross-linked collagen/chitosan porous scaffold for skin tissue engineering. Mater. Sci. Eng. C 2012, 32, 2361–2366. [Google Scholar] [CrossRef]
- Yannas, I.; Tzeranis, D.; So, P.T. Surface biology of collagen scaffold explains blocking of wound contraction and regeneration of skin and peripheral nerves. Biomed. Mater. 2015, 11, 014106. [Google Scholar] [CrossRef] [PubMed]
- Vojtová, L.; Zikmund, T.; Pavliňáková, V.; Šalplachta, J.; Kalasová, D.; Prosecká, E.; Brtníková, J.; Žídek, J.; Pavliňák, D.; Kaiser, J. The 3D imaging of mesenchymal stem cells on porous scaffolds using high-contrasted X-ary computed nanotomography. J. Microsc. 2019, 273, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Sloviková, A.; Vojtová, L.; Jančař, J. Preparation and modification of collagen-based porous scaffold for tissue engineering. Chem. Pap. 2008, 62, 417–422. [Google Scholar] [CrossRef]
- Hoque, M.E.; Nuge, T.; Yeow, T.K.; Nordin, N.; Prasad, R.G.S.V. Gelatin Based Scaffolds for Tissue Engineering—A Review. Polym. Res. J. 2015, 9, 15. [Google Scholar]
- Weyers, A.; Linhardt, R.J. Neoproteoglycans in tissue engineering. FEBS J. 2013, 280, 2511–2522. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Jang, T.-S.; Pan, H.M.; Jung, H.-D.; Sia, M.W.; Xie, S.; Hang, Y.; Chong, S.K.M.; Wang, D.; Song, J. 3D Freeform Printing of Nanocomposite Hydrogels through in situ Precipitation in Reactive Viscous Fluid. Int. J. Bioprint. 2020, 6, 258. [Google Scholar] [CrossRef]
- Farokhi, M.; Jonidi Shariatzadeh, F.; Solouk, A.; Mirzadeh, H. Alginate Based Scaffolds for Cartilage Tissue Engineering: A Review. Int. J. Polym. Mater. Polym. Biomater. 2020, 69, 230–247. [Google Scholar] [CrossRef]
- Ahmed, S.; Annu; Ali, A.; Sheikh, J. A review on chitosan centred scaffolds and their applications in tissue engineering. Int. J. Biol. Macromol. 2018, 116, 849–862. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, F.J. Biomaterials & scaffolds for tissue engineering. Mater. Today 2011, 14, 88–95. [Google Scholar]
- Chan, B.P.; Leong, K.W. Scaffolding in tissue engineering: General approaches and tissue-specific considerations. Eur. Spine J. 2008, 17, 467–479. [Google Scholar] [CrossRef] [Green Version]
- Nikolova, M.P.; Chavali, M.S. Recent advances in biomaterials for 3D scaffolds: A review. Bioact. Mater. 2019, 4, 271–292. [Google Scholar] [CrossRef]
- Gostynska, N.; Shankar Krishnakumar, G.; Campodoni, E.; Panseri, S.; Montesi, M.; Sprio, S.; Kon, E.; Marcacci, M.; Tampieri, A.; Sandri, M. 3D porous collagen scaffolds reinforced by glycation with ribose for tissue engineering application. Biomed. Mater. 2017, 12. [Google Scholar] [CrossRef]
- Reyna-Urrutia, V.A.; Mata-Haro, V.; Cauich-Rodriguez, J.V.; Herrera-Kao, W.A.; Cervantes-Uc, J.M. Effect of two crosslinking methods on the physicochemical and biological properties of the collagen-chitosan scaffolds. Eur. Polym. J. 2019, 117, 424–433. [Google Scholar] [CrossRef]
- Zhang, X.; Adachi, S.; Ura, K.; Takagi, Y. Properties of collagen extracted from Amur sturgeon Acipenser schrenckii and assessment of collagen fibrils in vitro. Int. J. Biol. Macromol. 2019, 137, 809–820. [Google Scholar] [CrossRef]
- Dong, C.; Lv, Y. Application of collagen scaffold in tissue engineering: Recent advances and new perspectives. Polymers 2016, 8, 42. [Google Scholar] [CrossRef] [Green Version]
- Sananta, P.; Rahaditya, I.G.M.O.; Imadudin, M.I.; Putera, M.A.; Andarini, S.; Kalsum, U.; Mustamsir, E.; Dradjat, R.S. Collagen scaffold for mesencyhmal stem cell from stromal vascular fraction (biocompatibility and attachment study): Experimental paper. Ann. Med. Surg. 2020, 59, 31–34. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarek, B.; Sionkowska, A. Chitosan/collagen blends with inorganic and organic additive—A review. Adv. Polym. Technol. 2018, 37, 2367–2376. [Google Scholar] [CrossRef]
- Babrnáková, J.; Pavliňáková, V.; Brtníková, J.; Sedláček, P.; Prosecká, E.; Rampichová, M.; Filová, E.; Hearnden, V.; Vojtová, L. Synergistic effect of bovine platelet lysate and various polysaccharides on the biological properties of collagen-based scaffolds for tissue engineering: Scaffold preparation, chemo-physical characterization, in vitro and ex ovo evaluation. Mater. Sci. Eng. C 2019, 100, 236–246. [Google Scholar] [CrossRef] [PubMed]
- Pratiwi, A.R.; Yuliati, A.; Soepribadi, I.; Ariani, M.D. Application of chitosan scaffolds on vascular endothelial growth factor and fibroblast growth factor 2 expressions in tissue engineering principles. Dent. J. 2015, 48, 213. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Wang, C.; Li, C.; Qin, Y.; Wang, Z.; Yang, F.; Li, Z.; Wang, J. A functional chitosan-based hydrogel as a wound dressing and drug delivery system in the treatment of wound healing. RSC Adv. 2018, 8, 7533–7549. [Google Scholar] [CrossRef] [Green Version]
- Dorazilová, J.; Muchová, J.; Šmerková, K.; Kočiová, S.; Diviš, P.; Kopel, P.; Veselý, R.; Pavliňáková, V.; Adam, V.; Vojtová, L. Synergistic Effect of Chitosan and Selenium Nanoparticles on Biodegradation and Antibacterial Properties of Collagenous Scaffolds Designed for Infected Burn Wounds. Nanomaterials 2020, 10, 1971. [Google Scholar] [CrossRef]
- Nečas, A.; Plánka, L.; Srnec, R.; Crha, M.; Hlučilová, J.; Klíma, J.; Starý, D.; Křen, L.; Amler, E.; Vojtová, L.; et al. Quality of Newly Formed Cartilaginous Tissue in Defects of Articular Surface after Transplantation of Mesenchymal Stem Cells in a Composite Scaffold Based on Collagen I with Chitosan Micro-and Nanofibres. Physiol. Res. 2010, 59, 605–614. [Google Scholar] [CrossRef]
- Peng, L.; Xiang, R.C.; Jia, W.W.; Dong, X.X.; Wang, G.E. Preparation and evaluation of porous chitosan/collagen scaffolds for periodontal tissue engineering. J. Bioact. Compat. Polym. 2006, 21, 207–220. [Google Scholar] [CrossRef]
- Tong, C.; Hao, H.; Xia, L.; Liu, J.; Ti, D.; Dong, L.; Hou, Q.; Song, H.; Liu, H.; Zhao, Y.; et al. Hypoxia pretreatment of bone marrow-derived mesenchymal stem cells seeded in a collagen-chitosan sponge scaffold promotes skin wound healing in diabetic rats with hindlimb ischemia. Wound Repair Regen. 2016, 24, 45–56. [Google Scholar] [CrossRef] [Green Version]
- Croisier, F.; Jérôme, C. Chitosan-based biomaterials for tissue engineering. Eur. Polym. J. 2013, 49, 780–792. [Google Scholar] [CrossRef] [Green Version]
- Muchová, J.; Hearnden, V.; Michlovská, L.; Vištejnová, L.; Zavad’áková, A.; Šmerková, K.; Kočiová, S.; Adam, V.; Kopel, P.; Vojtová, L. Mutual influence of selenium nanoparticles and FGF2-STAB® on biocompatible properties of collagen/chitosan 3D scaffolds: In vitro and ex ovo evaluation. J. Nanobiotechnol. 2021, 19, 103. [Google Scholar] [CrossRef] [PubMed]
- Dolivo, D.M.; Larson, S.A.; Dominko, T. Fibroblast Growth Factor 2 as an Antifibrotic: Antagonism of Myofibroblast Differentiation and Suppression of Pro-Fibrotic Gene Expression. Cytokine Growth Factor Rev. 2017, 38, 49–58. [Google Scholar] [CrossRef]
- Takei, Y.; Minamizaki, T.; Yoshiko, Y. Functional Diversity of Fibroblast Growth Factors in Bone Formation. Int. J. Endocrinol. 2015, 2015, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Acharya, A.N.; Coates, H.; Tavora-Vièira, D.; Rajan, G.P. A pilot study investigating basic fibroblast growth factor for the repair of chronic tympanic membrane perforations in pediatric patients. Int. J. Pediatr. Otorhinolaryngol. 2015, 79, 332–335. [Google Scholar] [CrossRef]
- Lou, Z.; Lou, Z. Regeneration of Traumatic Tympanic Membrane Perforations. Exp. Laryngol. Otol. 2017, 131, 564. [Google Scholar] [CrossRef] [PubMed]
- Coffin, J.D.; Homer-Bouthiette, C.; Hurley, M.M. Fibroblast Growth Factor 2 and Its Receptors in Bone Biology and Disease. J. Endocr. Soc. 2018, 2, 657–671. [Google Scholar] [CrossRef] [PubMed]
- Momose, T.; Miyaji, H.; Kato, A.; Ogawa, K.; Yoshida, T.; Nishida, E.; Murakami, S.; Kosen, Y.; Sugaya, T.; Kawanami, M. Collagen Hydrogel Scaffold and Fibroblast Growth Factor-2 Accelerate Periodontal Healing of Class II Furcation Defects in Dog. Open Dent. J. 2016, 10, 347–359. [Google Scholar] [CrossRef]
- Yoshida, T.; Miyaji, H.; Otani, K.; Inoue, K.; Nakane, K.; Nishimura, H.; Ibara, A.; Shimada, A.; Ogawa, K.; Nishida, E.; et al. Bone augmentation using a highly porous PLGA/β-TCP scaffold containing fibroblast growth factor-2. J. Periodontal Res. 2015, 50, 265–273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, W.; Cao, Y.; Zhang, Z.; Du, F.; Shi, Y.; Li, X.; Zhang, Q. Targeted delivery of FGF2 to subchondral bone enhanced the repair of articular cartilage defect. Acta Biomater. 2018, 69, 170–182. [Google Scholar] [CrossRef] [PubMed]
- Washio, A.; Teshima, H.; Yokota, K.; Kitamura, C.; Tabata, Y. Preparation of gelatin hydrogel sponges incorporating bioactive glasses capable for the controlled release of fibroblast growth factor-2. J. Biomater. Sci. Polym. Ed. 2019, 30, 49–63. [Google Scholar] [CrossRef] [PubMed]
- Fayazzadeh, E.; Yavarifar, H.; Rafie, S.R.; Motamed, S.; Sotoudeh Anvari, M.; Boroumand, M.A. Fibroblast Growth Factor-1 vs. Fibroblast Growth Factor-2 in Ischemic Skin Flap Survival in a Rat Animal Model. World J. Plast. Surg. 2016, 5, 274–279. [Google Scholar]
- Lin, W.; Xiang, L.-J.; Shi, H.-X.; Zhang, J.; Jiang, L.; Cai, P.; Lin, Z.-L.; Lin, B.-B.; Huang, Y.; Zhang, H.-L.; et al. Fibroblast Growth Factors Stimulate Hair Growth through β-Catenin and Shh Expression in C57BL/6 Mice. Biomed. Res. Int. 2015, 2015, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Ono, I. A Study on the Alterations in Skin Viscoelasticity before and after an Intradermal Administration of Growth Factor. J. Cutan. Aesthet. Surg. 2011, 4, 98–104. [Google Scholar] [CrossRef]
- Buchtova, M.; Chaloupkova, R.; Zakrzewska, M.; Vesela, I.; Cela, P.; Barathova, J.; Gudernova, I.; Zajickova, R.; Trantirek, L.; Martin, J.; et al. Instability restricts signaling of multiple fibroblast growth factors. Cell. Mol. Life Sci. 2015, 72, 2445–2459. [Google Scholar] [CrossRef]
- Dvorak, P.; Bednar, D.; Vanacek, P.; Balek, L.; Eiselleova, L.; Stepankova, V.; Sebestova, E.; Kunova Bosakova, M.; Konecna, Z.; Mazurenko, S.; et al. Computer-assisted engineering of hyperstable fibroblast growth factor 2. Biotechnol. Bioeng. 2018, 115, 850–862. [Google Scholar] [CrossRef]
- Prosecká, E.; Rampichová, M.; Litvinec, A.; Tonar, Z.; Králíčková, M.; Vojtová, L.; Kochová, P.; Plencner, M.; Buzgo, M.; Míčková, A.; et al. Collagen/hydroxyapatite scaffold enriched with polycaprolactone nanofibers, thrombocyte-rich solution and mesenchymal stem cells promotes regeneration in large bone defect in vivo. J. Biomed. Mater. Res. Part A 2015, 103, 671–682. [Google Scholar] [CrossRef]
- Vokurka, J.; Hromcik, F.; Faldyna, M.; Gopfert, E.; Vicenova, M.; Pozarova, L.; Holla, L.I. Platelet-rich plasma, platelet-rich fibrin, and enamel matrix derivative for oral mucosal wound healing. Pol. J. Vet. Sci. 2020, 23, 169–176. [Google Scholar] [CrossRef]
- Hromcik, F.; Vokurka, J.; Gopfert, E.; Faldyna, M.; Hermanova, M.; Kyr, M.; Vicenova, M.; Izakovicova Holla, L. Granulation tissue enriched by aspirin and omega-3 fatty acids in healing experimental periodontal lesion. Biomed. Pap. 2020. [Google Scholar] [CrossRef] [Green Version]
- Primer-BLAST. National Center for Biotechnology Information, Pike Bethesda, MD, USA. Available online: https://www.ncbi.nlm.nih.gov/tools/primer-blast/ (accessed on 27 January 2021).
- Zelnickova, P.; Matiasovic, J.; Pavlova, B.; Kudlackova, H.; Kovaru, F.; Faldyna, M. Quantitative nitric oxide production by rat, bovine and porcine macrophages. Nitric Oxide 2008, 19, 36–41. [Google Scholar] [CrossRef]
- Prosecká, E.; Rampichová, M.; Vojtová, L.; Tvrdík, D.; Melčáková, Š.; Juhasová, J.; Plencner, M.; Jakubová, R.; Jančář, J.; Nečas, A.; et al. Optimized conditions for mesenchymal stem cells to differentiate into osteoblasts on a collagen/hydroxyapatite matrix. J. Biomed. Mater. Res. Part A 2011, 99A, 307–315. [Google Scholar] [CrossRef]
- Zidek, J.; Vojtova, L.; Abdel-Mohsen, A.M.; Chmelik, J.; Zikmund, T.; Brtnikova, J.; Jakubicek, R.; Zubal, L.; Jan, J.; Kaiser, J. Accurate micro-computed tomography imaging of pore spaces in collagen-based scaffold. J. Mater. Sci. Mater. Med. 2016, 27, 110. [Google Scholar] [CrossRef]
- Bočková, J.; Vojtová, L.; Přikryl, R.; Čechal, J.; Jančář, J. Collagen-grafted ultra-high molecular weight polyethylene for biomedical applications. Chem. Pap. 2008, 62, 580–588. [Google Scholar] [CrossRef]
- Jančář, J.; Vojtová, L.; Nečas, A.; Srnec, R.; Urbanová, L.; Crha, M. Stability of collagen scaffold implants for animals with iatrogenic articular cartilage defects. Acta Vet-Brno 2009, 78, 643–648. [Google Scholar] [CrossRef]
- Andreopoulos, F.M.; Persaud, I. Delivery of basic fibroblast growth factor (bFGF) from photoresponsive hydrogel scaffolds. Biomaterials 2006, 27, 2468–2476. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.; Liu, Y.; Xiao, Z.S.; Prestwich, G.D. Injectable glycosaminoglycan hydrogels for controlled release of human basic fibroblast growth factor. Biomaterials 2005, 26, 6054–6067. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Gulbranson, D.R.; Yu, P.; Hou, Z.; Thomson, J.A. Thermal stability of fibroblast growth factor protein is a determinant factor in regulating self-renewal, differentiation, and reprogramming in human pluripotent stem cells. Stem Cells 2012, 30, 623–630. [Google Scholar] [CrossRef] [Green Version]
- Benington, L.; Rajan, G.; Locher, C.; Lim, L.Y. Fibroblast growth factor 2—A review of stabilisation approaches for clinical applications. Pharmaceutics 2020, 12, 508. [Google Scholar] [CrossRef] [PubMed]
- Tiede, S.; Ernst, N.; Bayat, A.; Paus, R.; Tronnier, V.; Zechel, C. Basic fibroblast growth factor: A potential new therapeutic tool for the treatment of hypertrophic and keloid scars. Ann. Anat. 2009, 191, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Akita, S.; Akino, K.; Hirano, A. Basic Fibroblast Growth Factor in Scarless Wound Healing. Adv. Wound Care 2013, 2, 44–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Liu, X.C.; Zhao, J.; Kong, X.R.; Shi, R.F.; Zhao, X.B.; Song, C.X.; Liu, T.J.; Lu, F. Degradable PLGA scaffolds with basic fibroblast growth factor: Experimental studies in myocardial revascularization. Tex. Heart Inst. J. 2009, 36, 89–97. [Google Scholar] [PubMed]
- Koledova, Z.; Sumbal, J.; Rabata, A.; de La Bourdonnaye, G.; Chaloupkova, R.; Hrdlickova, B.; Damborsky, J.; Stepankova, V. Fibroblast Growth Factor 2 Protein Stability Provides Decreased Dependence on Heparin for Induction of FGFR Signaling and Alters ERK Signaling Dynamics. Front. Cell Dev. Biol. 2019, 7, 331. [Google Scholar] [CrossRef] [Green Version]
- Ludwig, T.E.; Levenstein, M.E.; Jones, J.M.; Berggren, W.T.; Mitchen, E.R.; Frane, J.L.; Crandall, L.J.; Daigh, C.A.; Conard, K.R.; Piekarczyk, M.S.; et al. Derivation of human embryonic stem cells in defined conditions. Nat. Biotechnol. 2006, 24, 185–187. [Google Scholar] [CrossRef]
- Kanematsu, A.; Marui, A.; Yamamoto, S.; Ozeki, M.; Hirano, Y.; Yamamoto, M.; Ogawa, O.; Komeda, M.; Tabata, Y. Type I collagen can function as a reservoir of basic fibroblast growth factor. J. Control Release 2004, 99, 281–292. [Google Scholar] [CrossRef]
- Munisso, M.C.; Morimoto, N.; Notodihardjo, S.C.; Mitsui, T.; Kakudo, N.; Kusumoto, K. Collagen/Gelatin Sponges (CGSs) Provide Both Protection and Release of bFGF: An in Vitro Study. Biomed. Res. Int. 2019, 2019. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.M.; Xu, Y.Y.; Li, Z.H.; Yuan, X.Y.; Wang, P.F.; Zhang, X.Z.; Liu, Y.Q.; Guan, J.; Guo, Y.; Li, R.X.; et al. Heparin-functionalized collagen matrices with controlled release of basic fibroblast growth factor. J. Mater. Sci. Mater. Med. 2011, 22, 107–114. [Google Scholar] [CrossRef]
- Prasadh, S.; Wong, R.C.W. Unraveling the mechanical strength of biomaterials used as a bone scaffold in oral and maxillofacial defects. Oral Sci. Int. 2018, 15, 48–55. [Google Scholar] [CrossRef]
- Chen, Z.; Wei, B.; Mo, X.; Lim, C.T.; Ramakrishna, S.; Cui, F. Mechanical properties of electrospun collagen-chitosan complex single fibers and membrane. Mater. Sci. Eng. C 2009, 29, 2428–2435. [Google Scholar] [CrossRef]
- Susanto, A.; Satari, M.H.; Abbas, B.; Koesoemowidodo, R.S.A.; Cahyanto, A. Fabrication and Characterization of Chitosan-Collagen Membrane from Barramundi (Lates Calcarifer) Scales for Guided Tissue Regeneration. Eur. J. Dent. 2019, 13, 370–375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahmoud, A.A.; Salama, A.H. Norfloxacin-loaded collagen/chitosan scaffolds for skin reconstruction: Preparation, evaluation and in-vivo wound healing assessment. Eur. J. Pharm. Sci. 2016, 83, 155–165. [Google Scholar] [CrossRef]
- Rodríguez-Vázquez, M.; Vega-Ruiz, B.; Ramos-Zúñiga, R.; Saldaña-Koppel, D.A.; Quiñones-Olvera, L.F. Chitosan and Its Potential Use as a Scaffold for Tissue Engineering in Regenerative Medicine. Biomed. Res. Int. 2015, 2015, 821279. [Google Scholar] [CrossRef] [Green Version]
- Sionkowska, A.; Kaczmarek, B.; Gadzala-Kopciuch, R. Gentamicin release from chitosan and collagen composites. J. Drug Deliv. Sci. Technol. 2016, 35, 353–359. [Google Scholar] [CrossRef]
- Martínez, A.; Blanco, M.D.; Davidenko, N.; Cameron, R.E.; Martínez, A. Tailoring chitosan/collagen scaffolds for tissue engineering: Effect of composition 1 and different crosslinking agents on scaffold properties. Carbohydr. Polym. 2015, 132, 606–619. [Google Scholar] [CrossRef] [Green Version]
- Rezaii, M.; Oryan, S.; Javeri, A. Curcumin nanoparticles incorporated collagen-chitosan scaffold promotes cutaneous wound healing through regulation of TGF-β1/Smad7 gene expression. Mater. Sci. Eng. C 2019, 98, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Litwin, M.; Radwańska, A.; Paprocka, M.; Kieda, C.; Dobosz, T.; Witkiewicz, W.; Baczyńska, D. The role of FGF2 in migration and tubulogenesis of endothelial progenitor cells in relation to pro-angiogenic growth factor production. Mol. Cell. Biochem. 2015, 410, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Pepper, M.S.; Ferrara, N.; Orci, L.; Montesano, R. Potent synergism between vascular endothelial growth factor and basic fibroblast growth factor in the induction of angiogenesis in vitro. Biochem. Biophys. Res. Commun. 1992, 189, 824–831. [Google Scholar] [CrossRef]
- Seghezzi, G.; Patel, S.; Ren, C.J.; Gualandris, A.; Pintucci, G.; Robbins, E.S.; Shapiro, R.L.; Galloway, A.C.; Rifkin, D.B.; Mignatti, P. Fibroblast growth factor-2 (FGF-2) induces vascular endothelial growth factor (VEGF) expression in the endothelial cells of forming capillaries: An autocrine mechanism contributing to angiogenesis. J. Cell Biol. 1998, 141, 1659–1673. [Google Scholar] [CrossRef] [PubMed]
- Yun, Y.R.; Lee, S.; Jeon, E.; Kang, W.; Kim, K.H.; Kim, H.W.; Jang, J.H. Fibroblast growth factor 2-functionalized collagen matrices for skeletal muscle tissue engineering. Biotechnol. Lett. 2012, 34, 771–778. [Google Scholar] [CrossRef] [PubMed]
- Marks, M.G.; Doillon, C.; Silvert, F.H. Effects of fibroblasts and basic fibroblast growth factor on facilitation of dermal wound healing by type I collagen matrices. J. Biomed. Mater. Res. 1991, 25, 683–696. [Google Scholar] [CrossRef] [PubMed]
- Judith, R.; Nithya, M.; Rose, C.; Mandal, A.B. Application of a PDGF-containing novel gel for cutaneous wound healing. Life Sci. 2010, 87, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Nair, M.G. Macrophages in wound healing: Activation and plasticity. Immunol. Cell Biol. 2019, 97, 258–267. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, R.; Shukla, N. Attributes of alternatively activated (M2) macrophages. Life Sci. 2019, 224, 222–231. [Google Scholar] [CrossRef]
- Brew, K.; Dinakarpandian, D.; Nagase, H. Tissue inhibitors of metalloproteinases: Evolution, structure and function. Biochim. Biophys. Acta Protein Struct. Mol. Enzymol. 2000, 1477, 267–283. [Google Scholar] [CrossRef]
- Bertaux, B.; Hornebeck, W.; Eisen, A.Z.; Dubertret, L. Growth stimulation of human keratinocytes by tissue inhibitor of metalloproteinases. J. Investig. Dermatol. 1991, 97, 679–685. [Google Scholar] [CrossRef] [Green Version]
- Hayakawa, T.; Yamashita, K.; Tanzawa, K.; Uchijima, E.; Iwata, K. Growth-promoting activity of tissue inhibitor of metalloproteinases-1 (TIMP-1) for a wide range of cells A possible new growth factor in serum. FEBS Lett. 1992, 298, 29–32. [Google Scholar] [CrossRef] [Green Version]
- Holmes, K.; Roberts, O.L.; Thomas, A.M.; Cross, M.J. Vascular endothelial growth factor receptor-2: Structure, function, intracellular signalling and therapeutic inhibition. Cell. Signal. 2007, 19, 2003–2012. [Google Scholar] [CrossRef]
- Jetten, N.; Verbruggen, S.; Gijbels, M.J.; Post, M.J.; De Winther, M.P.J.; Donners, M.M.P.C. Anti-inflammatory M2, but not pro-inflammatory M1 macrophages promote angiogenesis in vivo. Angiogenesis 2014, 17, 109–118. [Google Scholar] [CrossRef]
- Singla, D.K.; Singla, R.D.; Abdelli, L.S.; Glass, C. Fibroblast growth factor-9 enhances M2 macrophage differentiation and attenuates adverse cardiac remodeling in the infarcted diabetic heart. PLoS ONE 2015, 10, e0120739. [Google Scholar] [CrossRef] [Green Version]
- Im, J.H.; Buzzelli, J.N.; Jones, K.; Franchini, F.; Gordon-Weeks, A.; Markelc, B.; Chen, J.; Kim, J.; Cao, Y.; Muschel, R.J. FGF2 alters macrophage polarization, tumour immunity and growth and can be targeted during radiotherapy. Nat. Commun. 2020, 11, 1–14. [Google Scholar] [CrossRef]
















| Sample Labeling | CFGF2 [µg/mL] | Pore Size [µm] |
|---|---|---|
| Coll/Chit | 0 | 240 ± 100 |
| Coll/Chit_0.01 FGF2 | 0.01 | 250 ± 100 |
| Coll/Chit_0.05 FGF2 | 0.05 | 240 ± 70 |
| Coll/Chit_0.1 FGF2 | 0.1 | 220 ± 60 |
| Coll/Chit_0.5 FGF2 | 0.5 | 220 ± 60 |
| Coll/Chit_1 FGF2 | 1 | 210 ± 60 |
| Coll/Chit_5 FGF2 | 5 | 180 ± 60 |
| Coll/Chit_10 FGF2 | 10 | 170 ± 90 |
| Coll/Chit_50 FGF2 | 50 | 170 ± 70 |
| Coll/Chit_100 FGF2 | 100 | 160 ± 60 |
| Gene | Forward Primer Reverse Primer | Function |
|---|---|---|
| TNF-α | CTCTGCCTCAGCCTCTTCTCTT | tumor-necrosis factor—alpha |
| AGGTTGTTTGGGGACTGCTCT | pro-inflammatory | |
| IL-1ß | ACAACAAGTGGTGTTCTCCATGA | Interleukin—1 beta |
| TTTCATCACGCAGACAGGTACA | pro-inflammatory | |
| IL-17 | ACCACATGAACTCTGTCCCAATC | Interleukin—17 |
| CCTACAGCCACCAGCATCTTC | pro-inflammatory | |
| MMP-9 | CACTGGGCTTGGATCACTCCTC | matrix metalloproteinasis—9 |
| GGGTTAGGACCATATAGATGCTGGA | pro-inflammatory | |
| IL-10 | TTCTGTGCCTGACCACACTTTC | Interleukin—10 |
| CTAGGAGTCTCTGGAACACTCGG | anti-inflammatory | |
| TIMP-1 | GTTTCTCATCGCTGGACAACTGC | tissue inhibitor of metalloproteinasis—1 |
| ACGAAACTGCAAGTCGTGATGTG | anti-inflammatory | |
| TGF-ß1 | TTCCCCTCCGAAAATGCCATCC | transforming growth factor—beta 1 |
| CACTCTGGCTTTTGGGTTCTGC | wound healing | |
| VEGF-C | GCTTCTTGTCTCTGGCGTGTTC | vascular endothelial growth factor—C |
| CCTACATAAGCCTTGGCCTCCTC | wound healing | |
| FGF-7 | ATCCTGCCAACTTTGCTCTACAGA | fibroblast growth factor—7 |
| CTGGAGTCATGTCATTGCAAGCT | wound healing | |
| HPRT-1 | TGAAACTGGAAAAGCAAATACAAAG | hypoxanthine phosphoribosyltransferase—1 |
| CGATGTCAATGAGACTCCTGATG | house-keeping gene | |
| GAPDH | GAATCCACTGGCGTCTTCAC | glyceraldehyde-3-phosphate dehydrogenase |
| CGTTGCTGACAATCTTGAGAGA | house-keeping gene | |
| HMBS | CAGCCATGAAGGATGGGCAGCTGTAC | hydroxymethylbilane synthase |
| TGCTGGCCTGCATGGTCTCTTGC | house-keeping gene |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vojtová, L.; Pavliňáková, V.; Muchová, J.; Kacvinská, K.; Brtníková, J.; Knoz, M.; Lipový, B.; Faldyna, M.; Göpfert, E.; Holoubek, J.; et al. Healing and Angiogenic Properties of Collagen/Chitosan Scaffolds Enriched with Hyperstable FGF2-STAB® Protein: In Vitro, Ex Ovo and In Vivo Comprehensive Evaluation. Biomedicines 2021, 9, 590. https://doi.org/10.3390/biomedicines9060590
Vojtová L, Pavliňáková V, Muchová J, Kacvinská K, Brtníková J, Knoz M, Lipový B, Faldyna M, Göpfert E, Holoubek J, et al. Healing and Angiogenic Properties of Collagen/Chitosan Scaffolds Enriched with Hyperstable FGF2-STAB® Protein: In Vitro, Ex Ovo and In Vivo Comprehensive Evaluation. Biomedicines. 2021; 9(6):590. https://doi.org/10.3390/biomedicines9060590
Chicago/Turabian StyleVojtová, Lucy, Veronika Pavliňáková, Johana Muchová, Katarína Kacvinská, Jana Brtníková, Martin Knoz, Břetislav Lipový, Martin Faldyna, Eduard Göpfert, Jakub Holoubek, and et al. 2021. "Healing and Angiogenic Properties of Collagen/Chitosan Scaffolds Enriched with Hyperstable FGF2-STAB® Protein: In Vitro, Ex Ovo and In Vivo Comprehensive Evaluation" Biomedicines 9, no. 6: 590. https://doi.org/10.3390/biomedicines9060590