Significance and Diagnostic Role of Antimicrobial Cathelicidins (LL-37) Peptides in Oral Health
Abstract
:1. Introduction
2. Types and Biochemistry of LL-37
- (1)
- N-terminal that is composed of 29–30 amino acid molecules and is assumed to guide the liberation of biologically active peptides;
- (2)
- cathelin-domain comprising of 98–114 amino acid molecules with its function not yet examined; and
- (3)
- C-terminal that comprises of 12–100 amino acid molecules as an active peptide with wide range of antimicrobial property against bacteria, viruses, and fungi.
- (1)
- LL-37 (leucine-leucine 37) that is found in humans [19],
- (2)
- CRAMP (cathelicidins related antimicrobial peptide) found in rats and mice [16],
- (3)
- Flowlicidin 1,2,3 and cathelicidins β-1 found in chickens [20],
- (4)
- CATH-1 and CATH-2 both are found in the Atlantic salmon [21],
- (5)
- p15s found in rodents, and CAP18 in rabbits [22], and
- (6)
- CAP11 is found in guinea pigs and LL-37 in rhesus monkeys [23].
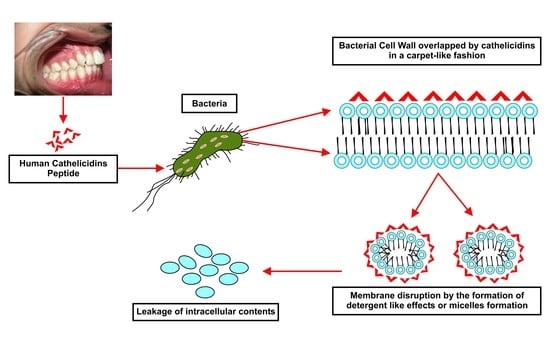
3. Mechanism of Action against Microbes
4. Importance of LL-37 in Oral Cavity
5. Diagnostic Biomarker in Oral Health and Research
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Dürr, U.H.N.; Sudheendra, U.S.; Ramamoorthy, A. LL-37, the only human member of the cathelicidin family of antimicrobial peptides. Biochim. Biophys. Acta Biomembr. 2006, 1758, 1408–1425. [Google Scholar] [CrossRef] [PubMed]
- Seo, M.D.; Won, H.S.; Kim, J.H.; Mishig-Ochir, T.; Lee, B.J. Antimicrobial peptides for therapeutic applications: A review. Molecules 2012, 17, 12276–12286. [Google Scholar] [CrossRef] [PubMed]
- Bandurska, K.; Berdowska, A.; Barczyńska-Felusiak, R.; Krupa, P. Unique features of human cathelicidin LL-37. BioFactors 2015, 41, 289–300. [Google Scholar] [CrossRef] [PubMed]
- Hilchie, A.L.; Wuerth, K.; Hancock, R.E.W. Immune modulation by multifaceted cationic host defense (antimicrobial) peptides. Nat. Chem. Biol. 2013, 9, 761–768. [Google Scholar] [CrossRef] [PubMed]
- Lehrer, R.I.; Ganz, T. Cathelicidins: A family of endogenous antimicrobial peptides. Curr. Opin. Hematol. 2002, 9, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Uzzell, T.; Stolzenberg, E.D.; Shinnar, A.E.; Zasloff, M. Hagfish intestinal antimicrobial peptides are ancient cathelicidins. Peptides 2003, 24, 1655–1667. [Google Scholar] [CrossRef] [PubMed]
- Gennaro, R.; Skerlavaj, B.; Romeo, D. Purification, composition, and activity of two bactenecins, antibacterial peptides of bovine neutrophils. Infect. Immun. 1989, 57, 3142–3146. [Google Scholar] [PubMed]
- Tomasinsig, L.; Zanetti, M. The cathelicidins—Structure, function and evolution. Curr. Protein Pept. Sci. 2005, 6, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Cowland, J.B.; Johnsen, A.H.; Borregaard, N. hCAP-18, a cathelin/pro-bactenecin-like protein of human neutrophil specific granules. FEBS Lett. 1995, 368, 173–176. [Google Scholar] [CrossRef]
- Sørensen, O.; Arnljots, K.; Cowland, J.B.; Bainton, D.F.; Borregaard, N. The human antibacterial cathelicidin, hCAP-18, is synthesized in myelocytes and metamyelocytes and localized to specific granules in neutrophils. Blood 1997, 90, 2796–2803. [Google Scholar] [PubMed]
- Agerberth, B.; Gunne, H.; Odeberg, J.; Kogner, P.; Boman, H.G.; Gudmundsson, G.H. FALL-39, a putative human peptide antibiotic, is cysteine-free and expressed in bone marrow and testis. Proc. Natl. Acad. Sci. USA 1995, 92, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Frohm, M.; Gunne, H.; Bergman, A.C.; Agerberth, B.; Bergman, T.; Boman, A.; Lidén, S.; Jörnvall, H.; Boman, H.G. Biochemical and antibacterial analysis of human wound and blister fluid. Eur. J. Biochem. 1996, 237, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Andersson, E.; Sørensen, O.E.; Frohm, B.; Borregaard, N.; Egesten, A.; Malm, J. Isolation of human cationic antimicrobial protein-18 from seminal plasma and its association with prostasomes. Hum. Reprod. 2002, 17, 2529–2534. [Google Scholar] [CrossRef] [PubMed]
- Murakami, M.; Ohtake, T.; Dorschner, R.A.; Gallo, R.L. Cathelicidin Antimicrobial Peptides are Expressed in Salivary Glands and Saliva. J. Dent. Res. 2002, 81, 845–850. [Google Scholar] [CrossRef] [PubMed]
- Türkoğlu, O.; Emingil, G.; Kütükçüler, N.; Atilla, G. Gingival Crevicular Fluid Levels of Cathelicidin LL-37 and Interleukin-18 in Patients With Chronic Periodontitis. J. Periodontol. 2009, 80, 969–976. [Google Scholar] [CrossRef] [PubMed]
- Gallo, R.L.; Kim, K.J.; Bernfield, M.; Kozak, C.A.; Zanetti, M.; Merluzzi, L.; Gennaro, R. Identification of CRAMP, a cathelin-related antimicrobial peptide expressed in the embryonic and adult mouse. J. Biol. Chem. 1997, 272, 13088–13093. [Google Scholar] [CrossRef] [PubMed]
- Agier, J.; Efenberger, M.; Brzezińska-Błaszczyk, E. Cathelicidin impact on inflammatory cells. Cent. Eur. J. Immunol. 2015, 40, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Prickett, M.D.; Gutowska, W.; Kuo, R.; Belov, K.; Burt, D.W. Evolution of the avian β-defensin and cathelicidin genes. BMC Evol. Biol. 2015, 15, 188. [Google Scholar] [CrossRef] [PubMed]
- Johansson, J.; Gudmundsson, G.H.; Rottenberg, M.E.; Berndt, K.D.; Agerberth, B. Conformation-dependent antibacterial activity of the naturally occurring human peptide LL-37. J. Biol. Chem. 1998, 273, 3718–3724. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Sunkara, L.T. Avian antimicrobial host defense peptides: From biology to therapeutic applications. Pharmaceuticals 2014, 7, 220–247. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-I.; Zhang, Y.-A.; Zou, J.; Nie, P.; Secombes, C.J. Two cathelicidin genes are present in both rainbow trout (Oncorhynchus mykiss) and atlantic salmon (Salmo salar). Antimicrob. Agents Chemother. 2006, 50, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Brock, R.; Luh, F.; Chou, P.J.; Larrick, J.W.; Huang, R.F.; Huang, T.H. The solution structure of the active domain of CAP18—A lipopolysaccharide binding protein from rabbit leukocytes. FEBS Lett. 1995, 370, 46–52. [Google Scholar] [CrossRef]
- Bals, R.; Lang, C.; Weiner, D.J.; Vogelmeier, C.; Welsch, U.; Wilson, J.M. Rhesus monkey (Macaca mulatta) mucosal antimicrobial peptides are close homologues of human molecules. Clin. Diagn. Lab. Immunol. 2001, 8, 370–375. [Google Scholar] [CrossRef] [PubMed]
- Mansour, S.C.; Pena, O.M.; Hancock, R.E.W. Host defense peptides: Front-line immunomodulators. Trends Immunol. 2014, 35, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Davidopoulou, S.; Diza, E.; Sakellari, D.; Menexes, G.; Kalfas, S. Salivary concentration of free LL-37 in edentulism, chronic periodontitis and healthy periodontium. Arch. Oral Biol. 2013, 58, 930–934. [Google Scholar] [CrossRef] [PubMed]
- Vandamme, D.; Landuyt, B.; Luyten, W.; Schoofs, L. A comprehensive summary of LL-37, the factoctum human cathelicidin peptide. Cell. Immunol. 2012, 280, 22–35. [Google Scholar] [CrossRef] [PubMed]
- Dale, B.A.; Tao, R.; Kimball, J.R.; Jurevic, R.J. Oral Antimicrobial Peptides and Biological Control of Caries. BMC Oral Health 2006, 6, S13. [Google Scholar] [CrossRef] [PubMed]
- Khurshid, Z.; Naseem, M.; Sheikh, Z.; Najeeb, S.; Shahab, S.; Zafar, M.S. Oral antimicrobial peptides: Types and role in the oral cavity. Saudi Pharm. J. 2015. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Mishra, B.; Epand, R.F.; Epand, R.M. High-quality 3D structures shine light on antibacterial, anti-biofilm and antiviral activities of human cathelicidin LL-37 and its fragments. Biochim. Biophys. Acta Biomembr. 2014, 1838, 2160–2172. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-C.; Park, Y.; Hahm, K.-S. The Role of Antimicrobial Peptides in Preventing Multidrug-Resistant Bacterial Infections and Biofilm Formation. Int. J. Mol. Sci. 2011, 12, 5971–5992. [Google Scholar] [CrossRef] [PubMed]
- Khurshid, Z.; Najeeb, S.; Mali, M.; Moin, S.F.; Raza, S.Q.; Zohaib, S.; Sefat, F.; Zafar, M.S. Histatin peptides: Pharmacological functions and its applications in dentistry. Saudi Pharm. J. 2016. [Google Scholar] [CrossRef]
- Ramamoorthy, A.; Lee, D.-K.; Narasimhaswamy, T.; Nanga, R.P.R. Cholesterol reduces pardaxin’s dynamics-a barrel-stave mechanism of membrane disruption investigated by solid-state NMR. Biochim. Biophys. Acta 2010, 1798, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-C.; Kim, J.-Y.; Shin, S.-O.; Jeong, C.-Y.; Kim, M.-H.; Shin, S.Y.; Cheong, G.-W.; Park, Y.; Hahm, K.-S. Investigation of toroidal pore and oligomerization by melittin using transmission electron microscopy. Biochem. Biophys. Res. Commun. 2006, 343, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Zaiou, M.; Nizet, V.; Gallo, R.L. Antimicrobial and protease inhibitory functions of the human cathelicidin (hCAP18/LL-37) prosequence. J. Investig. Dermatol. 2003, 120, 810–816. [Google Scholar] [CrossRef] [PubMed]
- Ståhle-bäckdahl, M.; Heilborn, J.; Carlsson, A.; Bogentoft, C. Use of the Catelicidin LL-37 and Derivatives Thereof for Wound Healing. U.S. Patent 7,452,864 B2, 18 November 2008. [Google Scholar]
- Haisma, E.M.; de Breij, A.; Chan, H.; van Dissel, J.T.; Drijfhout, J.W.; Hiemstra, P.S.; El Ghalbzouri, A.; Nibbering, P.H. LL-37-derived peptides eradicate multidrug-resistant Staphylococcus aureus from thermally wounded human skin equivalents. Antimicrob. Agents Chemother. 2014, 58, 4411–4419. [Google Scholar] [CrossRef] [PubMed]
- Gallo, R.; Murakami, M. Human Cathelicidin Antimicrobial Peptides. WO 2005040192 A3, 2005. [Google Scholar]
- Nagaoka, I.; Yomogida, S.; Tamura, H.; Hirata, M. Antibacterial cathelicidin peptide CAP11 inhibits the lipopolysaccharide (LPS)-induced suppression of neutrophil apoptosis by blocking the binding of LPS to target cells. Inflamm. Res. 2004, 53, 609–622. [Google Scholar] [CrossRef] [PubMed]
- Neumann, A.; Berends, E.T.; Nerlich, A.; Molhoek, E.M.; Gallo, R.L.; Meerloo, T.; Nizet, V.; Naim, H.Y.; von Kockritz-Blickwede, M. The antimicrobial peptide LL-37 facilitates the formation of neutrophil extracellular traps. Biochem. J. 2014, 464, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Cakir, E.; Torun, E.; Gedik, A.H.; Umutoglu, T.; Aktas, E.C.; Topuz, U.; Deniz, G. Cathelicidin and human β-defensin 2 in bronchoalveolar lavage fluid of children with pulmonary tuberculosis. Int. J. Tuberc. Lung Dis. 2014, 18, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Yoo, M.-W.; Park, J.; Han, H.-S.; Yun, Y.-M.; Kang, J.W.; Choi, D.-Y.; won Lee, J.; Jung, J.H.; Lee, K.-Y.; Kim, K.P. Discovery of gastric cancer-specific biomarkers by the application of serum proteomics. Proteomics 2017, 17, 1600332. [Google Scholar] [CrossRef] [PubMed]
- Morales, J.O.; Fathe, K.R.; Brunaugh, A.; Ferrati, S.; Li, S.; Montenegro-Nicolini, M.; Mousavikhamene, Z.; McConville, J.T.; Prausnitz, M.R.; Smyth, H.D.C. Challenges and Future Prospects for the Delivery of Biologics: Oral Mucosal, Pulmonary, and Transdermal Routes. AAPS J. 2017. [Google Scholar] [CrossRef] [PubMed]
- Potturu, M.; Prabhakaran, P.A.; Oommen, N.; Sarojini, D.M.; Sunil, S.N. Cathelicidin expression and role in oral health and diseases: A short review. Trop. J. Med. Res. 2014, 17, 69–75. [Google Scholar] [CrossRef]
- Mishra, A.; Apeksha, B.; Koppolu, P.; Lingam, S. Role of antimicrobial peptides in periodontal innate defense mechanism. J. Oral Res. Rev. 2015, 7, 74. [Google Scholar] [CrossRef]
- Barlow, P.G.; Svoboda, P.; Mackellar, A.; Nash, A.A.; York, I.A.; Pohl, J.; Davidson, D.J.; Donis, R.O. Antiviral Activity and Increased Host Defense against Influenza Infection Elicited by the Human Cathelicidin LL-37. PLoS ONE 2011, 6, e25333. [Google Scholar] [CrossRef] [PubMed]
- Gordon, Y.J.; Huang, L.C.; Romanowski, E.G.; Yates, K.A.; Proske, R.J.; McDermott, A.M. Human cathelicidin (LL-37), a multifunctional peptide, is expressed by ocular surface epithelia and has potent antibacterial and antiviral activity. Curr. Eye Res. 2005, 30, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.H.; Legowska, A.; Rolka, K.; Ng, T.B.; Hui, M.; Cho, C.H.; Lam, W.W.L.; Au, S.W.N.; Gu, O.W.; Wan, D.C.C. Effects of cathelicidin and its fragments on three key enzymes of HIV-1. Peptides 2011, 32, 1117–1122. [Google Scholar] [CrossRef] [PubMed]
- Khurshid, Z.; Najeeb, S.; Khan, R.S.; Zafar, M.S. Salivaomics: An Emerging Approach in Dentistry. JPDA 2016, 25, 41–43. [Google Scholar]
- Khurshid, Z.; Zohaib, S.; Najeeb, S.; Zafar, M.S.; Slowey, P.D.; Almas, K. Human Saliva Collection Devices for Proteomics: An Update. Int. J. Mol. Sci. 2016, 17, 846. [Google Scholar] [CrossRef] [PubMed]
- Khurshid, Z.; Zohaib, S.; Najeeb, S.; Zafar, M.; Rehman, R.; Rehman, I. Advances of Proteomic Sciences in Dentistry. Int. J. Mol. Sci. 2016, 17, 728. [Google Scholar] [CrossRef] [PubMed]
- Khurshid, Z.; Sohail Zafar, M.; Najeeb, S.; Zohaib, S. Human Saliva: A Future Diagnostic Tool. Dent. Sci. 2015, 2, 260–265. [Google Scholar] [CrossRef]
- Sannam Khan, R.; Khurshid, Z.; Akhbar, S.; Faraz Moin, S. Advances of Salivary Proteomics in Oral Squamous Cell Carcinoma (OSCC) Detection: An Update. Proteomes 2016, 4, 41. [Google Scholar] [CrossRef] [PubMed]
- Sahibzada, H.A.; Khurshid, Z.; Khan, R.S.; Naseem, M.; Siddique, K.M.; Mali, M.; Zafar, M.S. Salivary IL-8, IL-6 and TNF-α as Potential Diagnostic Biomarkers for Oral Cancer. Diagnostics 2017, 7, 21. [Google Scholar] [CrossRef] [PubMed]
- Khurshid, Z.; Mali, M.; Naseem, M.; Najeeb, S.; Zafar, M. Human Gingival Crevicular Fluids (GCF) Proteomics: An Overview. Dent. J. 2017, 5, 12. [Google Scholar] [CrossRef]
- Hans, M.; Hans, V.M. Epithelial Antimicrobial Peptides: Guardian of the Oral Cavity. Int. J. Pept. 2014, 2014, 370297. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Zhang, B.; Feng, X.; Ren, H.; Xu, J. Human cathelicidin LL-37 enhance the antibiofilm effect of EGCG on Streptococcus mutans. BMC Oral Health 2016, 16, 101. [Google Scholar] [CrossRef] [PubMed]
- Wiesner, J.; Vilcinskas, A. Antimicrobial peptides: The ancient arm of the human immune system. Virulence 2010, 1, 440–464. [Google Scholar] [CrossRef] [PubMed]
- Kajiya, M.; Shiba, H.; Komatsuzawa, H.; Ouhara, K.; Fujita, T.; Takeda, K.; Uchida, Y.; Mizuno, N.; Kawaguchi, H.; Kurihara, H. The antimicrobial peptide LL37 induces the migration of human pulp cells: A possible adjunct for regenerative endodontics. J. Endod. 2010, 36, 1009–1013. [Google Scholar] [CrossRef] [PubMed]
- Tsai, P.W.; Yang, C.Y.; Chang, H.T.; Lan, C.Y. Human antimicrobial peptide LL-37 inhibits adhesion of Candida albicans by interacting with yeast cell-wall carbohydrates. PLoS ONE 2011, 6, e17755. [Google Scholar] [CrossRef] [PubMed]
- Eick, S.; Puklo, M.; Adamowicz, K.; Kantyka, T.; Hiemstra, P.; Stennicke, H.; Guentsch, A.; Schacher, B.; Eickholz, P.; Potempa, J. Lack of cathelicidin processing in Papillon-Lefèvre syndrome patients reveals essential role of LL-37 in periodontal homeostasis. Orphanet J. Rare Dis. 2014, 9, 148. [Google Scholar] [CrossRef] [PubMed]
- Montreekachon, P.; Nongparn, S.; Sastraruji, T.; Khongkhunthian, S.; Chruewkamlow, N.; Kasinrerk, W.; Krisanaprakornkit, S. Favorable interleukin-8 induction in human gingival epithelial cells by the antimicrobial peptide LL-37. Asian Pac. J. Allergy Immunol. 2014, 32, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Davidopoulou, S.; Diza, E.; Menexes, G.; Kalfas, S. Salivary concentration of the antimicrobial peptide LL-37 in children. Arch. Oral Biol. 2012, 57, 865–869. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, Y.; Nagasawa, T.; Katagiri, S.; Kitagawara, S.; Kobayashi, H.; Koyanagi, T.; Izumi, Y. Salivary Levels of Antibacterial Peptide (LL-37/hCAP-18) and Cotinine in Patients With Chronic Periodontitis. J. Periodontol. 2012, 83, 766–772. [Google Scholar] [CrossRef] [PubMed]
- Davidopoulou, S.; Theodoridis, H.; Nazer, K.; Kessopoulou, E.; Menexes, G.; Kalfas, S. Salivary concentration of the antimicrobial peptide LL-37 in patients with oral lichen planus. J. Oral Microbiol. 2014, 6, 26156. [Google Scholar] [CrossRef] [PubMed]
- Gutner, M.; Chaushu, S.; Balter, D.; Bachrach, G. Saliva Enables the Antimicrobial Activity of LL-37 in the Presence of Proteases of Porphyromonas gingivalis. Infect. Immun. 2009, 77, 5558–5563. [Google Scholar] [CrossRef] [PubMed]
- Blodkamp, S.; Kadlec, K.; Gutsmann, T.; Naim, H.Y.; von Köckritz-Blickwede, M.; Schwarz, S. In vitro activity of human and animal cathelicidins against livestock-associated methicillin-resistant Staphylococcus aureus. Vet. Microbiol. 2015. [Google Scholar] [CrossRef] [PubMed]


| Peptide | Sequence | Staphylococcus aureus (MIC (µM)) | Escherichia coli (MIC (µM)) | Candida albicans (MIC (µM)) |
|---|---|---|---|---|
| LL-37 | LLGDFFRKSKEKIGKEFKRIVQRIKDFLRNLVPRTES-OH | >64 | 64 | 20 |
| RK31 | RKSKEKIGKEFKRIVQRIKDFLRNLVPRTES-OH | 16 | 8 | 4 |
| KS30 | KSKEKIGKEFKRIVQRIKDFLRNLVPRTES-OH | 16 | 8 | 2 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khurshid, Z.; Naseem, M.; Yahya I. Asiri, F.; Mali, M.; Sannam Khan, R.; Sahibzada, H.A.; Zafar, M.S.; Faraz Moin, S.; Khan, E. Significance and Diagnostic Role of Antimicrobial Cathelicidins (LL-37) Peptides in Oral Health. Biomolecules 2017, 7, 80. https://doi.org/10.3390/biom7040080
Khurshid Z, Naseem M, Yahya I. Asiri F, Mali M, Sannam Khan R, Sahibzada HA, Zafar MS, Faraz Moin S, Khan E. Significance and Diagnostic Role of Antimicrobial Cathelicidins (LL-37) Peptides in Oral Health. Biomolecules. 2017; 7(4):80. https://doi.org/10.3390/biom7040080
Chicago/Turabian StyleKhurshid, Zohaib, Mustafa Naseem, Faris Yahya I. Asiri, Maria Mali, Rabia Sannam Khan, Haafsa Arshad Sahibzada, Muhammad Sohail Zafar, Syed Faraz Moin, and Erum Khan. 2017. "Significance and Diagnostic Role of Antimicrobial Cathelicidins (LL-37) Peptides in Oral Health" Biomolecules 7, no. 4: 80. https://doi.org/10.3390/biom7040080