Improved Production and Biophysical Analysis of Recombinant Silicatein-α
Abstract
:1. Introduction
2. Materials and Methods
2.1. Gene Cloning and Protein Overexpression
2.2. Circular Dichroism
2.3. Dynamic Light Scattering, Static Light Scattering and Intrinsic Fluorescence
2.4. Enzyme Activity Assays
3. Results and Discussion
3.1. Design of Recombinant Protein Constructs
3.2. Optimisation of Silα Purification
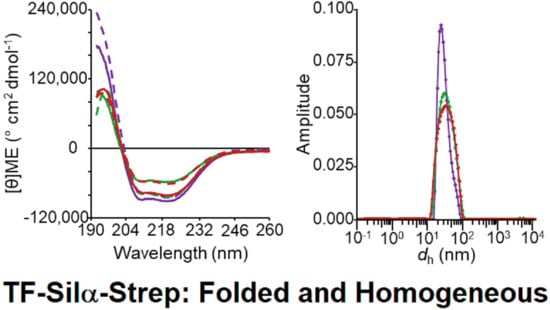
3.3. Circular Dichroism Spectroscopy
3.4. Dynamic Light Scattering
3.5. Temperature-Dependent Melting
3.6. Temperature-Dependent Aggregation
3.7. Enzymatic Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shimizu, K.; Cha, J.; Stucky, G.D.; Morse, D.E. Silicatein: Cathepsin L-like protein in sponge biosilica. Proc. Natl. Acad. Sci. USA 1998, 95, 6234–6238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brunner, E.; Gröger, C.; Lutz, K.; Richthammer, P.; Spinde, K.; Sumper, M. Analytical studies of silica biomineralization: Towards an understanding of silica processing by diatoms. Appl. Microbiol. Biotechnol. 2009, 84, 607–616. [Google Scholar] [CrossRef] [PubMed]
- Müller, W.E.G.; Krasko, A.; Le Pennec, G.; Steffen, R.; Wiens, M.; Ammar, M.S.A.; Müller, I.M.; Schröder, H.C. Molecular mechanism of spicule formation in the demosponge Suberites domuncula: Silicatein—collagen—myotrophin. Regul. Altern. Splic. 2003, 33, 195–221. [Google Scholar] [CrossRef]
- Brutchey, R.L.; E Morse, D. Silicatein and the Translation of its Molecular Mechanism of Biosilicification into Low Temperature Nanomaterial Synthesis. Chem. Rev. 2008, 108, 4915–4934. [Google Scholar] [CrossRef] [PubMed]
- Andriot, M.; Chao, S.H.; Colas, A.; Cray, S.; de Buyl, F.; DeGroot, J.V.; Dupont, A.; Easton, T.; Garaud, J.L.; Gerlach, E.; et al. Silicones in Industrial Applications. In Inorganic Polymers; De Jaeger, R., Gleria, M., Eds.; Nova Science Publishers: Hauppauge, NY, USA, 2007; pp. 61–161. [Google Scholar]
- Kung, M.C.; Riofski, M.V.; Missaghi, M.N.; Kung, H. Organosilicon platforms: Bridging homogeneous, heterogeneous, and bioinspired catalysis. Chem. Commun. 2014, 50, 3262–3276. [Google Scholar] [CrossRef]
- Rücker, C.; Kümmerer, K. Environmental Chemistry of Organosiloxanes. Chem. Rev. 2014, 115, 466–524. [Google Scholar] [CrossRef]
- Mewald, M.; Schiffner, J.A.; Oestreich, M. A New Direction in C–H Alkenylation: Silanol as a Helping Hand. Angew. Chem. Int. Ed. 2012, 51, 1763–1765. [Google Scholar] [CrossRef]
- Crouch, R. Recent Advances in Silyl Protection of Alcohols. Synth. Commun. 2013, 43, 2265–2279. [Google Scholar] [CrossRef]
- Xu, L.-W. Chiral Organosilicon Compounds. In Organosilicon Compounds; Elsevier BV: Amsterdam, The Netherlands, 2017; pp. 145–194. [Google Scholar] [CrossRef]
- Rösch, L.; John, P.; Reitmeier, R. Silicon Compounds, Organic. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley: Hoboken, NJ, USA, 2000; pp. 637–674. [Google Scholar] [CrossRef]
- Fairhead, M.; Johnson, K.A.; Kowatz, T.; McMahon, S.A.; Carter, L.G.; Oke, M.; Liu, H.; Naismith, J.H.; Van Der Walle, C. Crystal structure and silica condensing activities of silicatein α–cathepsin L chimeras. Chem. Commun. 2008, 1765–1767. [Google Scholar] [CrossRef] [Green Version]
- Murr, M.M.; Morse, D.E. Fractal intermediates in the self-assembly of silicatein filaments. Proc. Natl. Acad. Sci. USA 2005, 102, 11657–11662. [Google Scholar] [CrossRef] [Green Version]
- Godigamuwa, K.; Nakashima, K.; Okamoto, J.; Kawasaki, S. Biological Route to Fabricate Silica on Cellulose Using Immobilized Silicatein Fused with a Carbohydrate-Binding Module. Biomacromolecules 2020, 21, 2922–2928. [Google Scholar] [CrossRef] [PubMed]
- Dakhili, S.Y.T.; Caslin, S.A.; Faponle, A.S.; Quayle, P.; De Visser, S.P.; Wong, L.S. Recombinant silicateins as model biocatalysts in organosiloxane chemistry. Proc. Natl. Acad. Sci. USA 2017, 114, E5285–E5291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malhotra, A. Tagging for Protein Expression. Methods Enzymol. 2009, 463, 239–258. [Google Scholar] [CrossRef] [PubMed]
- Woestenenk, E.A.; Hammarström, M.; Berg, S.V.D.; Härd, T.; Berglund, H. His tag effect on solubility of human proteins produced in Escherichia coli: A comparison between four expression vectors. J. Struct. Funct. Genom. 2004, 5, 217–229. [Google Scholar] [CrossRef]
- Wu, J.; Filutowicz, M. Hexahistidine (His6)-tag dependent protein dimerization: A cautionary tale. Acta Biochim. Pol. 1999, 46, 591–599. [Google Scholar] [CrossRef] [Green Version]
- Schoonen, L.; Van Esterik, K.S.; Zhang, C.; Ulijn, R.V.; Nolte, R.J.M.; Van Hest, J.C. Alternative application of an affinity purification tag: Hexahistidines in ester hydrolysis. Sci. Rep. 2017, 7, 14772. [Google Scholar] [CrossRef] [Green Version]
- Fonda, I.; Kenig, M.; Gaberc-Porekar, V.; Pristovaek, P.; Menart, V. Attachment of Histidine Tags to Recombinant Tumor Necrosis Factor-Alpha Drastically Changes Its Properties. Sci. World J. 2002, 2, 1312–1325. [Google Scholar] [CrossRef] [Green Version]
- Freydank, A.-C.; Brandt, W.; Dräger, B. Protein structure modeling indicates hexahistidine-tag interference with enzyme activity. Proteins Struct. Funct. Bioinform. 2008, 72, 173–183. [Google Scholar] [CrossRef]
- Kim, Y.; Bigelow, L.; Borovilos, M.; Dementieva, I.; Duggan, E.; Eschenfeldt, W.; Hatzos, C.; Joachimiak, G.; Li, H.; Maltseva, N.; et al. High-Throughput Protein Purification for X-Ray Crystallography and NMR. Adv. Protein Chem. Struct. Biol. 2008, 75, 85–105. [Google Scholar] [CrossRef] [Green Version]
- Narmandakh, A.; Bearne, S.L. Purification of recombinant mandelate racemase: Improved catalytic activity. Protein Expr. Purif. 2010, 69, 39–46. [Google Scholar] [CrossRef]
- Aitken, A.; Learmonth, M.P.; Walker, J.M. Protein Determination by UV Absorption. In Protein Protocols Handbook; John, M.W., Ed.; Humana Press: Totowa, NJ, USA, 2002; pp. 3–6. [Google Scholar] [CrossRef]
- Gasteiger, E.; Gattiker, A.; Hoogland, C.; Ivanyi, I.; Appel, R.D.; Bairoch, A. ExPASy: The proteomics server for in-depth protein knowledge and analysis. Nucleic Acids Res. 2003, 31, 3784–3788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aphalo, P.J. Ggpmisc: Miscellaneous Extensions to ’Ggplot2’. R Package Version 0.3.3. Available online: https://CRAN.R-project.org/package=ggpmisc (accessed on 19 August 2020).
- Wilkins, D.K.; Grimshaw, S.B.; Receveur, V.; Dobson, C.M.; Jones, J.A.; Smith, L.J. Hydrodynamic radii of native and denatured proteins measured by pulse field gradient NMR techniques. Biochemistry 1999, 38, 16424–16431. [Google Scholar] [CrossRef] [PubMed]
- Skerra, A.; Schmidt, T.G. Use of the Strep-Tag and streptavidin for detection and purification of recombinant proteins. Methods Enzymol. 2000, 326, 271–304. [Google Scholar] [CrossRef] [PubMed]
- Korndörfer, I.P.; Skerra, A. Improved affinity of engineered streptavidin for the Strep-tag II peptide is due to a fixed open conformation of the lid-like loop at the binding site. Protein Sci. 2002, 11, 883–893. [Google Scholar] [CrossRef] [PubMed]
- Rosenow, M.A.; Magee, C.L.; Williams, J.C.; Allen, J.P. The influence of detergents on the solubility of membrane proteins. Acta Crystallogr. Sect. D Boil. Crystallogr. 2002, 58, 2076–2081. [Google Scholar] [CrossRef] [Green Version]
- Golovanov, A.P.; Hautbergue, G.M.; Wilson, S.A.; Lian, L.-Y. A Simple Method for Improving Protein Solubility and Long-Term Stability. J. Am. Chem. Soc. 2004, 126, 8933–8939. [Google Scholar] [CrossRef]
- Arakawa, T.; Ejima, D.; Tsumoto, K.; Obeyama, N.; Tanaka, Y.; Kita, Y.; Timasheff, S.N. Suppression of protein interactions by arginine: A proposed mechanism of the arginine effects. Biophys. Chem. 2007, 127, 1–8. [Google Scholar] [CrossRef]
- Tischer, A.; Lilie, H.; Rudolph, R.; Lange, C. L-Arginine hydrochloride increases the solubility of folded and unfolded recombinant plasminogen activator rPA. Protein Sci. 2010, 19, 1783–1795. [Google Scholar] [CrossRef] [Green Version]
- Bjellqvist, B.; Basse, B.; Olsen, E.; Celis, J.E. Reference points for comparisons of two-dimensional maps of proteins from different human cell types defined in a pH scale where isoelectric points correlate with polypeptide compositions. Electrophoresis 1994, 15, 529–539. [Google Scholar] [CrossRef]
- McCue, J.T. Chapter 25 Theory and Use of Hydrophobic Interaction Chromatography in Protein Purification Applications. Methods Enzymol. 2009, 463, 405–414. [Google Scholar] [CrossRef]
- Wen, J.; Arakawa, T.; Philo, J.S. Size-Exclusion Chromatography with On-Line Light-Scattering, Absorbance, and Refractive Index Detectors for Studying Proteins and Their Interactions. Anal. Biochem. 1996, 240, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Keller, D.J.; Bustamante, C. Theory of the interaction of light with large inhomogeneous molecular aggregates. II. Psi-type circular dichroism. J. Chem. Phys. 1986, 84, 2972–2980. [Google Scholar] [CrossRef]
- Patzelt, H.; Kramer, G.; Rauch, T.; Schonfeld, H.-J.; Bukau, B.; Deuerling, E. Three-State Equilibrium of Escherichia coli Trigger Factor. Boil. Chem. 2002, 383, 1611–1619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoffmann, A.; Bukau, B.; Kramer, G. Structure and function of the molecular chaperone Trigger Factor. Biochim. Biophys. Acta (BBA) Bioenerg. 2010, 1803, 650–661. [Google Scholar] [CrossRef] [Green Version]
- Stetefeld, J.; McKenna, S.A.; Patel, T.R. Dynamic light scattering: A practical guide and applications in biomedical sciences. Biophys. Rev. 2016, 8, 409–427. [Google Scholar] [CrossRef]
- International Organization for Standardization. Particle Size Analysis—Dynamic Light Scattering (Dls); International Organization for Standardization: London, UK, 2008. [Google Scholar]
- Rogošić, M.; Mencer, H.; Gomzi, Z. Polydispersity index and molecular weight distributions of polymers. Eur. Polym. J. 1996, 32, 1337–1344. [Google Scholar] [CrossRef]
- Panek, A.; Pietrow, O.; Filipkowski, P.; Synowiecki, J. Effects of the polyhistidine tag on kinetics and other properties of trehalose synthase from Deinococcus geothermalis. Acta Biochim. Pol. 2013, 60, 163–166. [Google Scholar] [CrossRef]
- Cha, J.N.; Shimizu, K.; Zhou, Y.; Christiansen, S.C.; Chmelka, B.F.; Stucky, G.D.; Morse, D.E. Silicatein filaments and subunits from a marine sponge direct the polymerization of silica and silicones in vitro. Proc. Natl. Acad. Sci. USA 1999, 96, 361–365. [Google Scholar] [CrossRef] [Green Version]
- Patwardhan, S.V.; Clarson, S.J. Silicification and Biosilicification. Part 6. Poly-L-Histidine Mediated Synthesis of Silica at Neutral pH. J. Inorg. Organomet. Polym. 2003, 13, 49–53. [Google Scholar] [CrossRef]
- Kuno, T.; Nonoyama, T.; Hirao, K.; Kato, K. Influence of the Charge Relay Effect on the Silanol Condensation Reaction as a Model for Silica Biomineralization. Langmuir 2011, 27, 13154–13158. [Google Scholar] [CrossRef]
- Maraite, A.; Ansörge-Schumacher, M.B.; Ganchegui, B.; Leitner, W.; Grogan, G. On the biocatalytic cleavage of silicon–oxygen bonds: A substrate structural approach to investigating the cleavage of protecting group silyl ethers by serine-triad hydrolases. J. Mol. Catal. B Enzym. 2009, 56, 24–28. [Google Scholar] [CrossRef]
- Higueruelo, A.; Schreyer, A.; Bickerton, G.R.J.; Pitt, W.R.; Groom, C.R.; Blundell, T.L. Atomic Interactions and Profile of Small Molecules Disrupting Protein-Protein Interfaces: The TIMBAL Database. Chem. Boil. Drug Des. 2009, 74, 457–467. [Google Scholar] [CrossRef] [PubMed]







| Entry | Buffer a | Additives b | pH | Presence of Soluble Protein After Lysis c | IMAC Compatible d |
|---|---|---|---|---|---|
| 1 | Tris | NaCl, L-Arg, L-Glu | 8.5 | × | n.d. |
| 2 | EPPS | NaCl, L-Arg, L-Glu | 8.5 | × | n.d. |
| 3 | BisTris | NaCl, L-Arg, L-Glu | 8.5 | × | n.d. |
| 4 | CHES | NaCl, L-Arg, L-Glu | 9.5 | √ | × |
| 5 | Tris | NaCl, CHAPS (5 mM), Triton X-100 (1%) | 8.5 | √ | × |
| 6 | CHES | NaCl | 9.5 | √ | × |
| 7 e | Tris | NaCl | 7.5 | × | n.d. |
| 8 | Tris | NaCl | 8.5 | √ | √ |
| 9 | AMP | NaCl | 8.5 | √ | × |
| 10 | AMP | NaCl | 9.0 | √ | × |
| 11 | AMP | NaCl | 9.5 | √ | × |
| 12 | AMP | NaCl | 10.0 | √ | × |
| 13 | AMP | NaCl | 10.5 | √ | × |
| Entry | Buffer a | Additives a | pH | Presence of Soluble Protein After Lysis b | SAC Compatible c |
|---|---|---|---|---|---|
| 1 | CHES | NaCl, L-Arg, L-Glu | 9.5 | √ | √ |
| 2 | Tris | NaCl, CHAPS (5 mM), Triton X-100 (1%) | 8.5 | √ | √ |
| 3 | CHES | NaCl | 9.5 | √ | √ |
| 4 | Tris | NaCl | 8.5 | √ | √ |
| 5 | AMP | NaCl | 8.5 | √ | √ |
| 6 | AMP | NaCl | 9.0 | √ | √ |
| 7 | AMP | NaCl | 9.5 | √ | √ |
| 8 | AMP | NaCl | 10.0 | √ | √ |
| 9 | AMP | NaCl | 10.5 | √ | √ |
| Buffer | Additives | pH |
|---|---|---|
| Potassium phosphate (100 mM) | KCl (20 mM) | 7.0 |
| Tris-HCl (50 mM) | NaCl (100 mM) | 8.5 |
| AMP (50 mM) | NaCl (100 mM) | 9.0 |
| Protein | Buffer | pH | Isolated Yield (nmol L−1 of Cell Culture) a |
|---|---|---|---|
| His6-Silα | Tris (50 mM), NaCl (100 mM) | 8.5 | 8 |
| His6-TF- Silα | Potassium phosphate (100 mM) | 8.0 | 6 |
| Tris (50 mM), NaCl (100 mM) | 8.5 | 177 | |
| TF-Silα-Strep | Tris (50 mM), NaCl (100 mM) | 8.5 | 777 |
| AMP (50 mM), NaCl (100 mM) | 9.0 | 1540 |
| pH | His6-TF-Silα | TF-Silα-Strep | ||
|---|---|---|---|---|
| % Polydispersity | PDI | % Polydispersity | PDI | |
| 7.0 | 93.8 ± 27.3 | 1.65 ± 0.57 | 41.6 ± 6.4 | 0.326 ± 0.047 |
| 8.5 | 130.3 ± 15.6 | 2.29 ± 0.07 | 49.3 ± 1.7 | 0.302 ± 0.062 |
| 9.0 | 61.7 ± 21.5 | 1.47 ± 0.68 | 34.5 ± 6.7 | 0.152 ± 0.079 |
| Enzyme | KM (µM) | kcat (min−1) | kcat/KM (min−1 µM−1) |
|---|---|---|---|
| His6-TF-Silα | 22.4 ± 2.2 a | 988 ± 416 a | 44.1 |
| TF-Silα-Strep | 88.2 ± 34.6 | 0.150 ± 0.033 | 0.00170 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sparkes, E.I.; Kettles, R.A.; Egedeuzu, C.S.; Stephenson, N.L.; Caslin, S.A.; Tabatabaei Dakhili, S.Y.; Wong, L.S. Improved Production and Biophysical Analysis of Recombinant Silicatein-α. Biomolecules 2020, 10, 1209. https://doi.org/10.3390/biom10091209
Sparkes EI, Kettles RA, Egedeuzu CS, Stephenson NL, Caslin SA, Tabatabaei Dakhili SY, Wong LS. Improved Production and Biophysical Analysis of Recombinant Silicatein-α. Biomolecules. 2020; 10(9):1209. https://doi.org/10.3390/biom10091209
Chicago/Turabian StyleSparkes, Emily I., Rachel A. Kettles, Chisom S. Egedeuzu, Natalie L. Stephenson, Stephanie A. Caslin, S. Yasin Tabatabaei Dakhili, and Lu Shin Wong. 2020. "Improved Production and Biophysical Analysis of Recombinant Silicatein-α" Biomolecules 10, no. 9: 1209. https://doi.org/10.3390/biom10091209