Climate and Wolbachia Impacts on Anoplolepis gracilipes (Hymenoptera: Formicidae)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
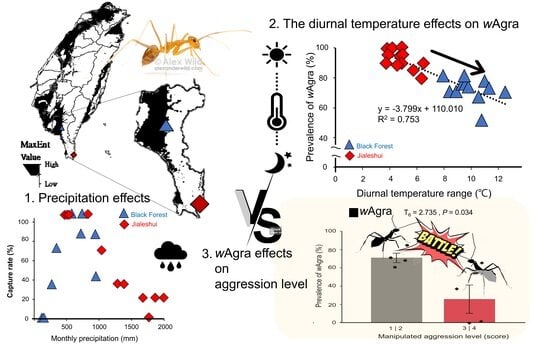
3.1. MaxEnt Modeling and Climate Factor Selection
3.2. Regression Analysis among Modeling, Climate Data, and Field Surveys
3.3. The Aggression Analysis and Prevalence of wAgra
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mooney, H.A. Invasive Alien Species: A New Synthesis, 3rd ed.; Island Press: Washington, DC, USA, 2005; pp. 59–83. [Google Scholar]
- Lee, C.Y.; Yang, C.C.S. Biology, ecology, and management of the invasive longlegged ant, Anoplolepis gracilipes. Annu. Rev. Entomol. 2022, 67, 43–63. [Google Scholar] [CrossRef]
- Angulo, E.; Hoffmann, B.D.; Ballesteros-Mejia, L.; Taheri, A.; Balzani, P.; Bang, A.; Courchamp, F. Economic costs of invasive alien ants worldwide. Biol. Invasions 2022, 24, 2041–2060. [Google Scholar] [CrossRef]
- Hoffmann, B.D.; Saul, W.C. Yellow crazy ant (Anoplolepis gracilipes) invasions within undisturbed mainland Australian habitats: No support for biotic resistance hypothesis. Biol. Invasions 2010, 12, 3093–3108. [Google Scholar] [CrossRef]
- Kiritani, K. Different effects of climate change on the population dynamics of insects. Appl. Entomol. Zool. 2013, 48, 97–104. [Google Scholar] [CrossRef]
- Bertelsmeier, C.; Luque, G.M.; Hoffmann, B.D.; Courchamp, F. Worldwide ant invasions under climate change. Biodivers. Conserv. 2015, 24, 117–128. [Google Scholar] [CrossRef]
- Morrison, L.W.; Michael, D.K.; Sanford, D.P. Predicted range expansion of the invasive fire ant, Solenopsis invicta, in the eastern United States based on the VEMAP global warming scenario. Divers. Distrib. 2005, 11, 199–204. [Google Scholar] [CrossRef]
- Chen, S.; Ding, F.; Hao, M.; Jiang, D. Mapping the potential global distribution of red imported fire ant (Solenopsis invicta Buren) based on a machine learning method. Sustainability 2020, 12, 10182. [Google Scholar] [CrossRef]
- Warren, R.J.; Chick, L.D. Upward ant distribution shift corresponds with minimum, not maximum, temperature tolerance. Glob. Chang. Biol. 2013, 19, 2082–2088. [Google Scholar] [CrossRef]
- Warren, R.J.; Candeias, M.; Lafferty, A.; Chick, L.D. Regional-scale environmental resistance to non-native ant invasion. Biol Invasions 2020, 22, 813–825. [Google Scholar] [CrossRef]
- Dunn, R.R.; Agosti, D.; Andersen, A.N.; Arnan, X.; Bruhl, C.A.; Cerdá, X.; Sanders, N.J. Climatic drivers of hemispheric asymmetry in global patterns of ant species richness. Ecol. Lett. 2009, 12, 324–333. [Google Scholar] [CrossRef]
- Heller, N.E.; Sanders, N.J.; Shors, J.W.; Gordon, D.M. Rainfall facilitates the spread, and time alters the impact, of the invasive Argentine ant. Oecologia 2008, 155, 385–395. [Google Scholar] [CrossRef]
- Jung, J.M.; Jung, S.; Ahmed, M.R.; Cho, B.K.; Lee, W.H. Invasion risk of the yellow crazy ant Anoplolepis gracilipes under the Representative Concentration Pathways 8.5 climate change scenario in South Korea. J. Asia-Pac. Biodivers. 2017, 10, 548–554. [Google Scholar] [CrossRef]
- Lee, W.H.; Song, J.W.; Yoon, S.H.; Jung, J.M. Spatial evaluation of machine learning-based species distribution models for prediction of invasive ant species distribution. Appl. Sci. 2022, 12, 10260. [Google Scholar] [CrossRef]
- Bertelsmeier, C.; Blight, O.; Courchamp, F. Invasions of ants (Hymenoptera: Formicidae) in light of global climate change. Myrmecol. News 2016, 22, 25–42. [Google Scholar]
- Cooling, M.; Hartley, S.; Sim, D.A.; Lester, P.J. The widespread collapse of an invasive species: Argentine ants (Linepithema humile) in New Zealand. Biol Lett. 2012, 8, 430–433. [Google Scholar] [CrossRef]
- Morrison, L.W.; Porter, S.D.; Daniels, E.; Korzukhin, M.D. Potential Global Range Expansion of the Invasive Fire Ant, Solenopsis invicta. Biol. Invasions 2004, 6, 183–191. [Google Scholar] [CrossRef]
- Araújo, M.B.; Guisan, A. Five (or so) challenges for species distribution modelling. J. Biogeogr. 2006, 33, 1677–1688. [Google Scholar] [CrossRef]
- Lemoine, M.M.; Engl, T.; Kaltenpoth, M. Microbial symbionts expanding or constraining abiotic niche space in insects. Curr. Opin. Insect. Sci. 2020, 39, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Richard, F.J. Symbiotic bacteria influence the odor and mating preference of their hosts. Front. Ecol. Evol. 2017, 5, 143. [Google Scholar] [CrossRef]
- Hedges, L.M.; Brownlie, J.C.; O’Neill, S.L.; Johnson, K.N. Wolbachia and virus protection in insects. Science 2008, 322, 702. [Google Scholar] [CrossRef]
- Werren, J.H.; Baldo, L.; Clark, M.E. Wolbachia: Master manipulators of invertebrate biology. Nat. Rev. Microbiol. 2008, 6, 741–751. [Google Scholar] [CrossRef] [PubMed]
- Russell, J.A. The ants (Hymenoptera: Formicidae) are unique and enigmatic hosts of prevalent Wolbachia (Alphaproteobacteria) symbionts. Myrmecol. News 2012, 16, 7–23. [Google Scholar]
- Wenseleers, T.; Ito, F.; van Borm, S.; Huybrechts, R.; Volckaert, F.; Billen, J. Widespread occurrence of the microorganism Wolbachia in ants. Proc. Biol. Sci. 1998, 265, 1447–1452. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Linksvayer, T.A. Wolbachia-infected ant colonies have increased reproductive investment and an accelerated life cycle. J. Exp. Biol. 2020, 223, jeb220079. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.; Chen, S.; Huang, Y.; Pierce, N.E.; Riegler, M.; Yang, F.; Xu, Y. Symbiotic microbiota may reflect host adaptation by resident to invasive ant species. PLOS Pathog. 2019, 15, e1007942. [Google Scholar] [CrossRef] [PubMed]
- Pontieri, L.; Schmidt, A.M.; Singh, R.; Pedersen, J.S.; Linksvayer, T.A. Artificial selection on ant female caste ratio uncovers a link between female-biased sex ratios and infection by Wolbachia endosymbionts. J. Evol. Biol. 2017, 30, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Sebastien, A.; Gruber, M.A.M.; Lester, P.J. Prevalence and genetic diversity of three bacterial endosymbionts Wolbachia, Arsenophonus, and Rhizobiales associated with the invasive yellow crazy ant Anoplolepis gracilipes. Insectes Soc. 2012, 59, 33–40. [Google Scholar] [CrossRef]
- Charlesworth, J.; Weinert, L.A.; Araujo Jr, E.V.; Welch, J.J. Wolbachia, Cardinium and climate: An analysis of global data. Biol. Lett. 2019, 15, 20190273. [Google Scholar] [CrossRef]
- Avtaeva, T.; Petrovičová, K.; Langraf, V.; Brygadyrenko, V. Potential bioclimatic ranges of crop pests Zabrus tenebrioides and Harpalus rufipes during climate change conditions. Diversity 2021, 13, 559. [Google Scholar] [CrossRef]
- Kriticos, D.J.; Webber, B.L.; Leriche, A.; Ota, N.; Macadam, I.; Bathols, J.; Scott, J.K. CliMond: Global high-resolution historical and future scenario climate surfaces for bioclimatic modelling. Methods Ecol. Evol. 2012, 3, 53–64. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Modell. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Elith, J.; Phillips, S.J.; Hastie, T.; Dudík, M.; Chee, Y.Y.; Yates, C.J. A statistical explanation of MAXENT for ecologists. Divers. Distrib. 2011, 17, 43–57. [Google Scholar] [CrossRef]
- Calenge, C. The package ‘adhabitat’ for the R software: A tool for the analysis of space and habitat use by animals. Ecol. Modell. 2006, 197, 516–519. [Google Scholar] [CrossRef]
- Hirzel, A.H.; Hausser, J.; Chessel, D.; Perrin, N. Ecological-niche factor analysis: How to compute habitat-suitability maps without absence data? Ecology 2002, 83, 2027–2036. [Google Scholar] [CrossRef]
- Hill, M.P.; Axford, J.; Hoffmann, A.A. Predicting the spread of Aedes albopictus in Australia under current and future climates: Multiple approaches and datasets to incorporate potential evolutionary divergence. Austral Ecol. 2014, 39, 469–478. [Google Scholar] [CrossRef]
- Rödder, D.; Lötters, S. Niche shift versus niche conservatism? Climatic characteristics of the native and invasive ranges of the Mediterranean house gecko (Kemidactylus turcicus). Glob. Ecol. Biogeogr. 2009, 18, 674–687. [Google Scholar] [CrossRef]
- Suarez, A.V.; Tsutsui, N.D.; Holway, D.A.; Case, T.J. Behavioral and genetic differentiation between native and introduced populations of the Argentine Ant. Biol. Invasions 1999, 1, 43–53. [Google Scholar] [CrossRef]
- Hsu, H.W.; Chiu, M.C.; Lee, C.C.; Lee, C.Y.; Yang, C.S. The association between virus prevalence and intercolonial aggression levels in the yellow crazy ant, Anoplolepis Gracilipes (Jerdon). Insects 2019, 10, 436. [Google Scholar] [CrossRef]
- PubMLST. Available online: https://pubmlst.org/wolbachia/info/protocols.shtml (accessed on 26 August 2017).
- Zhou, W.; Rousset, F.; O’Neill, S. Phylogeny and PCR–based classification of Wolbachia strains using wsp gene sequences. Proc. R Soc. Lond. Ser. B Biol. Sci. 1998, 265, 509–515. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023; Available online: http://r-project.org (accessed on 21 September 2023).
- Pearce, J.; Ferrier, S. Evaluating the predictive performance of habitat models developed using logistic regression. Ecol. Modell. 2000, 133, 225–245. [Google Scholar] [CrossRef]
- Lee, C.-C.; Lin, C.-Y.; Tseng, S.-P.; Matsuura, K.; Yang, C.-C.S. Ongoing coevolution of Wolbachia and a widespread invasive ant, Anoplolepis gracilipes. Microorganisms 2020, 8, 1569. [Google Scholar] [CrossRef]
- Tseng, S.-P.; Hsu, P.-W.; Lee, C.-C.; Wetterer, J.K.; Hugel, S.; Wu, L.-H.; Lee, C.-Y.; Yoshimura, T.; Yang, C.-C.S. Evidence for common horizontal transmission of Wolbachia among ants and ant crickets: Kleptoparasitism. Microorganisms 2020, 8, 805. [Google Scholar] [CrossRef] [PubMed]
- Mertl, A.L.; Ryder Wilkie, K.T.; Traniello, J.F. Impact of flooding on the species richness, density and composition of Amazonian litter-nesting ants. Biotropica 2009, 41, 633–641. [Google Scholar] [CrossRef]
- Kolay, S.; Annagiri, S. Dual response to nest flooding during monsoon in an Indian ant. Sci. Rep. 2015, 5, 13716. [Google Scholar] [CrossRef] [PubMed]
- Haines, I.H.; Haines, J.B. Colony structure, seasonality and food requirements of the crazy ant, Anoplolepis longipes (Jerd.), in the Seychelles. Ecol. Entomol. 1978, 3, 109–118. [Google Scholar] [CrossRef]
- Corbin, C.; Heyworth, E.R.; Ferrari, J.; Hurst, G.D. Heritable symbionts in a world of varying temperature. Heredity 2017, 118, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Jia, F.X.; Yang, M.S.; Yang, W.J.; Wang, J.J. Influence of continuous high temperature conditions on Wolbachia infection frequency and the fitness of Liposcelis tricolor (Psocoptera: Liposcelididae). Environ. Entomol. 2009, 38, 1365–1372. [Google Scholar] [CrossRef] [PubMed]
- Hague, M.T.J.; Caldwell, C.N.; Cooper, B.S. Pervasive effects of Wolbachia on host temperature preference. mBio 2020, 6, e01768-20. [Google Scholar] [CrossRef]
- Wang, L.; Jiang, J.; Xu, Y.; Zeng, L.; Lu, Y. Occurrence of three intracellular symbionts (Wolbachia, Arsenophonus, Cardinium) among ants in southern China. J. Asia-Pac. Entomol. 2016, 19, 981–988. [Google Scholar] [CrossRef]
- Rohrscheib, C.E.; Bondy, E.; Josh, P.; Riegler, M.; Eyles, D.; van Swinderen, B.; Wedell, N. Wolbachia influences the production of octopamine and affects Drosophila male aggression. Appl. Environ. Microbiol. 2015, 81, 4573–4580. [Google Scholar] [CrossRef]
- Sprenger, P.P.; Burkert, L.H.; Abou, B.; Federle, W.; Menzel, F. Coping with the climate: Cuticular hydrocarbon acclimation of ants under constant and fluctuating conditions. J. Exp. Biol. 2018, 221, jeb171488. [Google Scholar] [CrossRef] [PubMed]





| Jialeshui | March | April | May | June | July | August | September | October | November | December | January | February | March |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| March | — | 1.20 ± 0.13 c | 1.00 ± 0.00 c | 1.70 ± 0.30 bc | 2.90 ± 0.23 a | 2.70 ± 0.26 ab | |||||||
| April | — | 1.30 ± 0.15 bc | 2.20 ± 0.36 ab | 2.40 ± 0.27 a | 2.10 ± 0.31 ab | ||||||||
| May | — | 2.10 ± 0.28 a | 2.60 ± 0.31 a | 2.20 ± 0.13 a | 2.30 ± 0.26 a | 1.70 ± 0.21 a | |||||||
| June | — | 1.40 ± 0.16 b | 1.30 ± 0.15 b | 3.00 ± 0.26 a | 2.50 ± 0.25 a | 1.30 ± 0.21 b | |||||||
| July | — | 1.50 ± 0.15 bc | 2.30 ± 0.14 a | 2.50 ± 0.20 a | 1.20 ± 0.12 c | 2.00 ± 0.19 ab | |||||||
| August | — | 2.90 ± 0.28 a | 2.60 ± 0.22 ab | 1.70 ± 0.21 b | 1.80 ± 0.29 ab | 2.00 ± 0.30 ab | 1.90 ± 0.31 ab | 2.00 ± 0.21 ab | |||||
| September | — | 1.70 ± 0.15a | 1.10 ± 0.10 a | 1.70 ± 0.21 a | 1.50 ± 0.17 a | 1.70 ± 0.21 a | 1.70 ± 0.26 a | ||||||
| October | — | 1.30 ± 0.15 a | 1.50 ± 0.17 a | 1.40 ± 0.22 a | |||||||||
| November | — | 1.00 ± 0.00 b | 1.40 ± 0.16 ab | 1.30 ± 0.15 ab | 1.70 ± 0.21 a | ||||||||
| December | — | 1.20 ± 0.13 a | 1.70 ± 0.14 a | 1.20 ± 0.13 a | |||||||||
| January | — | 1.10 ± 0.10 a | 1.40 ± 0.16 a | ||||||||||
| February | — | 1.10 ± 0.10 | |||||||||||
| Black Forest | March | April | May | June | July | August | September | October | November | December | January | February | March |
| March | — | 1.70 ± 0.19 a | 1.90 ± 0.21 a | 2.20 ± 0.23 a | 1.50 ± 0.15 a | 1.90 ± 0.16 a | 1.80 ± 0.12 a | 1.60 ± 0.15 a | 1.90 ± 0.25 a | 1.80 ± 0.18 a | 2.20 ± 0.23 a | 1.90 ± 0.16 a | |
| April | — | 1.20 ± 0.13 a | 1.10 ± 0.10 a | 1.40 ± 0.16 a | 1.30 ± 0.15 a | 1.40 ± 0.22 a | |||||||
| May | — | 1.00 ± 0.00 a | 1.10 ± 0.09 a | 1.70 ± 0.27 a | 1.60 ± 0.20 a | ||||||||
| June | — | 1.30 ± 0.15 a | 1.00 ± 0.00 a | 1.20 ± 0.13 a | 1.80 ± 0.33 a | ||||||||
| July | — | 1.00 ± 0.00 a | 1.30 ± 0.15 a | 1.20 ± 0.13 a | 1.20 ± 0.20 a | 1.30 ± 0.15 a | 1.10 ± 0.10 a | ||||||
| August | — | 1.20 ± 0.13 a | 1.40 ± 0.16 a | 1.70 ± 0.33 b | |||||||||
| September | — | 1.30 ± 0.15 a | 1.10 ± 0.10 a | 1.50 ± 0.22 a | 1.70 ± 0.30 a | ||||||||
| October | — | 1.60 ± 0.22 a | 1.30 ± 0.15 a | 1.20 ± 0.13 a | |||||||||
| November | — | 1.10 ± 0.10 a | 1.00 ± 0.00 a | 1.40 ± 0.16 a | |||||||||
| December | — | 1.20 ± 0.13 a | 1.30 ± 0.15 a | 1.50 ± 0.16 a | |||||||||
| January | — | 1.20 ± 0.13 a | 1.20 ± 0.13 a | ||||||||||
| February | — | 1.00 ± 0.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, Y.-J.; Yeh, C.-H.; Wu, C.-Z.; Wu, L.-H. Climate and Wolbachia Impacts on Anoplolepis gracilipes (Hymenoptera: Formicidae). Biology 2023, 12, 1482. https://doi.org/10.3390/biology12121482
Lin Y-J, Yeh C-H, Wu C-Z, Wu L-H. Climate and Wolbachia Impacts on Anoplolepis gracilipes (Hymenoptera: Formicidae). Biology. 2023; 12(12):1482. https://doi.org/10.3390/biology12121482
Chicago/Turabian StyleLin, Yu-Jen, Ching-Hong Yeh, Chen-Zhe Wu, and Li-Hsin Wu. 2023. "Climate and Wolbachia Impacts on Anoplolepis gracilipes (Hymenoptera: Formicidae)" Biology 12, no. 12: 1482. https://doi.org/10.3390/biology12121482