Phyto-Synthesis, Characterization, and In Vitro Antibacterial Activity of Silver Nanoparticles Using Various Plant Extracts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Plant Extracts
2.3. Synthesis of Silver Nanoparticles
2.4. Characterizations of Silver Nanoparticles
2.5. Comparative Study of Mung Bean (Vigna radiata L.) Responses towards Ag-NPs and Their Salt
2.6. Seed Sterilization and Germination
2.7. Treatments
2.8. Plant Length
2.9. Root and Shoot Weight
2.10. In Vitro Susceptibility, Minimum Inhibitory Concentration (MIC), and Minimum Bactericidal Concentration (MBC) Evaluation of Ag-NPs
3. Results
3.1. UV–Visible Spectroscopy
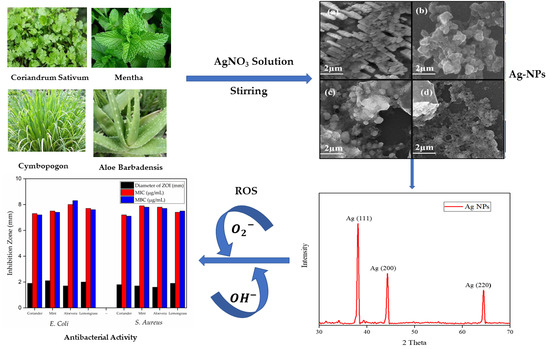
3.2. Field Emission Scanning Electron Microscopy (FESEM)
3.3. Energy-Dispersive X-ray Spectroscopy (EDX)
3.4. Particle Size Analysis (PSA)
3.5. Thermogravimetric Analysis Curve
3.6. XRD Analysis
3.7. Comparative Study of Mung Bean (Vigna radiata L.) Responses towards Ag-NPs and Their Salt
3.8. In Vitro Susceptibility, Minimum Inhibitory Concentration (MIC), and Minimum Bactericidal Concentration (MBC) Evaluation of Ag-NPs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Uzair, B.; Liaqat, A.; Iqbal, H.; Menaa, B.; Razzaq, A.; Thiripuranathar, G.; Fatima Rana, N.; Menaa, F. Green and Cost-Effective Synthesis of Metallic Nanoparticles by Algae: Safe Methods for Translational Medicine. Bioengineeing 2020, 7, 129. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Yadav, S.C.; Yadav, S.K. Syzygium cumini leaf and seed extract mediated biosynthesis of silver nanoparticles and their characterization. J. Chem. Technol. Biotechnol. 2010, 85, 1301–1309. [Google Scholar] [CrossRef]
- Ali, M.A.; Rehman, I.; Iqbal, A.; Din, S.; Rao, A.Q.; Latif, A.; Samiullah, T.R.; Azam, S.; Husnain, T. Nanotechnology, a new frontier in Agriculture. Adv. Life Sci. 2014, 1, 129–138. [Google Scholar]
- Chernousova, S.; Epple, M. Silver as antibacterial agent: Ion, nanoparticle, and metal. Angew. Chem. Int. Ed. Engl. 2013, 52, 1636–1653. [Google Scholar] [CrossRef] [PubMed]
- Elumalai, E.; Prasad, T.; Hemachandran, J.; Therasa, S.V.; Thirumalai, T.; David, E. Extracellular synthesis of silver nanoparticles using leaves of Euphorbia hirta and their antibacterial activities. J. Pharm. Sci. Res. 2010, 2, 549–554. [Google Scholar]
- Krishnaraj, C.; Jagan, E.; Rajasekar, S.; Selvakumar, P.; Kalaichelvan, P.; Mohan, N. Synthesis of silver nanoparticles using Acalypha indica leaf extracts and its antibacterial activity against water borne pathogens. Colloids Surf. B Biointerfaces 2010, 76, 50–56. [Google Scholar] [CrossRef]
- Prasad, T.; Elumalai, E. Biofabrication of Ag nanoparticles using Moringa oleifera leaf extract and their antimicrobial activity. Asian Pac. J. Trop. Biomed. 2011, 1, 439–442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ravindra, S.; Mohan, Y.M.; Reddy, N.N.; Raju, K.M. Fabrication of antibacterial cotton fibres loaded with silver nanoparticles via “Green Approach”. Colloids Surf. A Physico chem Eng. Asp. 2010, 367, 31–40. [Google Scholar] [CrossRef]
- Begum, N.A.; Mondal, S.; Basu, S.; Laskar, R.A.; Mandal, D. Biogenic synthesis of Au and Ag nanoparticles using aqueous solutions of Black Tea leaf extracts. Colloids Surf. B Biointerfaces 2009, 71, 113–118. [Google Scholar] [CrossRef]
- Vickers, N.J. Animal communication: When i’m calling you, will you answer too? Curr. Biol. 2017, 27, R713–R715. [Google Scholar] [CrossRef]
- Gardea-Torresdey, J.L.; Parsons, J.; Gomez, E.; Peralta-Videa, J.; Troiani, H.; Santiago, P.; Yacaman, M.J. Formation and growth of Au nanoparticles inside live alfalfa plants. Nano Lett. 2002, 2, 397–401. [Google Scholar] [CrossRef]
- Wijnhoven, S.W.; Peijnenburg, W.J.; Herberts, C.A.; Hagens, W.I.; Oomen, A.G.; Heugens, E.H.; Roszek, B.; Bisschops, J.; Gosens, I.; Van De Meent, D. Nano-silver—A review of available data and knowledge gaps in human and environmental risk assessment. Nanotoxicology 2009, 3, 109–138. [Google Scholar] [CrossRef]
- Scheringer, M.; Macleod, M.; Behra, T.; Sigg, L.; Hungerbühler, K. Environmental risks associated with nanoparticulate silver used as biocide. Househ. Pers. Care Today 2010, 1, 34–37. [Google Scholar]
- Taghavizadeh Yazdi, M.E.; Darroudi, M.; Amiri, M.S.; Zarrinfar, H.; Hosseini, H.A.; Mashreghi, M.; Mozafarri, H.; Ghorbani, A.; Mousavi, S.H. Antimycobacterial, anticancer, antioxidant and photocatalytic activity of biosynthesized silver nanoparticles using berberis integerrima. Iran J. Sci. Technol. Trans. A. Sci. 2022, 46, 1–11. [Google Scholar] [CrossRef]
- Ommati, M.M.; Jamshidzadeh, A.; Heidari, R.; Sun, Z.; Zamiri, M.J.; Khodaei, F.; Mousapour, S.; Ahmadi, F.; Javanmard, N.; Shirazi Yeganeh, B. Carnosine and histidine supplementation blunt lead-induced reproductive toxicity through antioxidative and mitochondria-dependent mechanisms. Biol. Trace Elem. Res. 2019, 187, 151–162. [Google Scholar] [CrossRef]
- Maensiri, S.; Laokul, P.; Klinkaewnarong, J.; Phokha, S.; Promarak, V.; Seraphin, S. Indium oxide (In2O3) nanoparticles using Aloe vera plant extract: Synthesis and optical properties. J. Optoelectron. Adv. Mater. 2008, 10, 161–165. [Google Scholar]
- Zhu, H.; Wang, N.; Wang, L.; Yao, K.; Shen, X. In situ x-ray diffraction study of the phase transition of nanocrystalline In (OH)3 to In2O3. Inorg. Mater. 2005, 41, 609–612. [Google Scholar] [CrossRef]
- Sharif, M.; Tunio, S.A.; Bano, S. Synergistic effects of Zinc oxide nanoparticles and conventional antibiotics against methicillin resistant Staphylococcus aureus. Adv. Life Sci. 2021, 8, 167–171. [Google Scholar]
- Zhang, Y.; Ago, H.; Liu, J.; Yumura, M.; Uchida, K.; Ohshima, S.; Iijima, S.; Zhu, J.; Zhang, X. The synthesis of In, In2O3 nanowires and In2O3 nanoparticles with shape controlled. J. Cryst. Growth. 2004, 264, 363–368. [Google Scholar] [CrossRef]
- Lao, J.; Huang, J.; Wang, D.; Ren, Z. Self-assembled In2O3 nanocrystal chains and nanowire networks. Adv. Mater. 2004, 16, 65–69. [Google Scholar] [CrossRef]
- Caballero, B.; Trugo, L.C.; Finglas, P.M. Encyclopedia of Food Sciences and Nutrition; Academic Press: Cambridge, MA, USA, 2003. [Google Scholar]
- Qu, J.; Yuan, X.; Wang, X.; Shao, P. Zinc accumulation and synthesis of ZnO nanoparticles using Physalis alkekengi L. Environ. Pollut. 2011, 159, 1783–1788. [Google Scholar] [CrossRef] [PubMed]
- De Sousa Barros, A.; de Morais, S.M.; Ferreira, P.A.T.; Vieira, Í.G.P.; Craveiro, A.A.; dos Santos Fontenelle, R.O.; de Menezes, J.E.S.A.; da Silva, F.W.F.; de Sousa, H.A. Chemical composition and functional properties of essential oils from Mentha species. Ind. Crop. Prod. 2015, 76, 557–564. [Google Scholar] [CrossRef]
- Qu, J.; Luo, C.; Hou, J. Synthesis of ZnO nanoparticles from Zn-hyperaccumulator (Sedum alfredii Hance) plants. Micro Nano Lett. 2011, 6, 174–176. [Google Scholar] [CrossRef]
- Manen, J.-F.; Sinitsyna, O.; Aeschbach, L.; Markov, A.V.; Sinitsyn, A. A fully automatable enzymatic method for DNA extraction from plant tissues. BMC Plant Biol. 2005, 5, 23. [Google Scholar] [CrossRef] [Green Version]
- Nalimu, F.; Oloro, J.; Kahwa, I.; Ogwang, P.E. Review on the phytochemistry and toxicological profiles of Aloe vera and Aloe ferox. Future J. Pharm. Sci. 2021, 7, 145. [Google Scholar] [CrossRef]
- Fiore, V.; Badagliacco, D.; Sanfilippo, C.; Pirrone, R.; Siengchin, S.; Rangappa, S.M.; Botta, L. Lemongrass Plant as Potential Sources of Reinforcement for Biocomposites: A Preliminary Experimental Comparison between Leaf and Culm Fibers. J. Polym. Environ. 2022, 30, 4726–4737. [Google Scholar] [CrossRef]
- Yang, X.; Li, Q.; Wang, H.; Huang, J.; Lin, L.; Wang, W.; Sun, D.; Su, Y.; Opiyo, J.B.; Hong, L. Green synthesis of palladium nanoparticles using broth of Cinnamomum camphora leaf. J. Nanopart. Res. 2010, 12, 1589–1598. [Google Scholar] [CrossRef] [Green Version]
- Rehman, K.U.; Hamayun, M.; Khan, S.A.; Khan, S.S.; Wali, S. Competence of benzoil tree (Moringa oleifera L.) as antibacterial and antifungal agent. Adv. Life Sci. 2020, 7, 135–139. [Google Scholar]
- Das, S.; Kalita, M.C.; Shukla, S. Rapid biosynthesis of silver nanoparticles using leaf extract of Brassica oleracea Var. Gongylodes and their antimicrobial activity against bacteria. World J. Pharm. Pharm. Sci. 2018, 7, 1135–1145. [Google Scholar]
- Yuvasree, P.; Nithya, K.; Neelakandeswari, N. Biosynthesis of silver nanoparticles from Aloe vera plant extract and its antimicrobial activity against multidrug resistant pathogens. In Proceedings of the International Conference on Advanced Nanomaterials & Emerging Engineering Technologies, Chennai, India, 24–26 July 2013; pp. 84–86. [Google Scholar]
- Masurkar, S.A.; Chaudhari, P.R.; Shidore, V.B.; Kamble, S.P. Rapid biosynthesis of silver nanoparticles using Cymbopogan citratus (lemongrass) and its antimicrobial activity. Nano-Micro Lett. 2011, 3, 189–194. [Google Scholar] [CrossRef] [Green Version]
- Sathyavathi, R.; Krishna, M.B.; Rao, S.V.; Saritha, R.; Rao, D.N. Biosynthesis of silver nanoparticles using Coriandrum sativum leaf extract and their application in nonlinear optics. Adv. Sci. Lett. 2010, 3, 138–143. [Google Scholar] [CrossRef] [Green Version]
- Bauer, A.W.; Kirby, W.M.; Sherris, J.C.; Turck, M. Antibiotic susceptibility testing by a standardized single disk method. Am. J. Clin. Pathol. 1966, 45, 493–496. [Google Scholar] [CrossRef]
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing; Twenty-Second Informational Supplement; The Clinical and Laboratory Standards Institute (CLSI): Wayne, PA, USA, 2012. [Google Scholar]
- Chou, K.-S.; Lai, Y.-S. Effect of polyvinyl pyrrolidone molecular weights on the formation of nanosized silver colloids. Mater. Chem. Phys. 2004, 83, 82–88. [Google Scholar] [CrossRef]
- Mustafa, F.; Razwan, M.; Shabbir, S. Microstructure and resistivity analysis of silver nanoparticle-based crystalline conductive films synthesized using PEG surfactant. Processes 2019, 7, 245. [Google Scholar] [CrossRef] [Green Version]
- Sondi, I.; Salopek-Sondi, B. Silver nanoparticles as antimicrobial agent: A case study on E. coli as a model for Gram-negative bacteria. J. Colloid Interface Sci. 2004, 275, 177–182. [Google Scholar] [CrossRef]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef] [PubMed]
- Emamhadi, M.A.; Sarafraz, M.; Akbari, M.; Fakhri, Y.; Linh, N.T.T.; Khaneghah, A.M. Nanomaterials for food packaging applications: A systematic review. Food Chem. Toxicol. 2020, 146, 111825. [Google Scholar] [CrossRef] [PubMed]
- Zarei, M.; Jamnejad, A.; Khajehali, E. Antibacterial effect of silver nanoparticles against four foodborne pathogens. Jundishapur J. Microbiol. 2014, 7, 8720–8723. [Google Scholar] [CrossRef] [Green Version]
- Al-Sarraj, F.M. A Review on the impacts of Azadirachta indica on Multi-drug Resistant Extended Spectrum Beta Lactamase-positive of Escherichia coli and Klebsiella pneumonia. Adv. Life Sci. 2021, 8, 228–232. [Google Scholar]
- Hamayun, M.; Khan, S.S.; Ahmad, N.; Wali, S. Efficiency of Virgin’s Mantle (Fagonia cretica L.) as an Antibacterial and Antifungal Agent. Adv. Life Sci. 2021, 8, 233–237. [Google Scholar]
- Ali, N.; Afrasiab, H.; Anwar, S. Antibacterial activity of leaf extracts of seven grape cultivars against six strains of bacteria. Adv. Life Sci. 2019, 6, 159–164. [Google Scholar]
- Salunke, B.K.; Sathiyamoorthi, E.; Tran, T.K.; Kim, B.S. Phyto-synthesized silver nanoparticles for biological applications. Korean J. Chem. Eng. 2017, 34, 943–951. [Google Scholar] [CrossRef]
- Ma, X.; Geiser-Lee, J.; Deng, Y.; Kolmakov, A. Interactions between engineered nanoparticles (ENPs) and plants: Phytotoxicity, uptake and accumulation. Sci. Total Envion. 2010, 408, 3053–3061. [Google Scholar] [CrossRef] [PubMed]
- Mirzajani, F.; Askari, H.; Hamzelou, S.; Farzaneh, M.; Ghassempour, A. Effect of silver nanoparticles on Oryza sativa L. and its rhizosphere bacteria. Ecotoxicol. Environ. Saf. 2013, 88, 48–54. [Google Scholar] [CrossRef]
- Geisler-Lee, J.; Wang, Q.; Yao, Y.; Zhang, W.; Geisler, M.; Li, K.; Huang, Y.; Chen, Y.; Kolmakov, A.; Ma, X. Phytotoxicity, accumulation and transport of silver nanoparticles by Arabidopsis thaliana. Nanotoxicology 2012, 7, 323–337. [Google Scholar] [CrossRef] [PubMed]
- Gardea-Torresdey, J.L.; Rico, C.M.; White, J.C. Trophic transfer, transformation, and impact of engineered nanomaterials in terrestrial environments. Environ. Sci. Technol. 2014, 48, 2526–2540. [Google Scholar] [CrossRef] [PubMed]
- Oukarroum, A.; Barhoumi, L.; Pirastru, L.; Dewez, D. Silver nanoparticle toxicity effect on growth and cellular viability of the aquatic plant Lemna gibba. Environ. Toxicol. Chem. 2013, 32, 902–907. [Google Scholar] [CrossRef] [PubMed]
- Thuesombat, P.; Hannongbua, S.; Akasit, S.; Chadchawan, S. Effect of silver nanoparticles on rice (Oryza sativa L. cv. KDML 105) seed germination and seedling growth. Ecotoxicol. Environ. Saf. 2014, 104, 302–309. [Google Scholar] [CrossRef]
- Cvjetko, P.; Milošić, A.; Domijan, A.-M.; Vrček, I.V.; Tolić, S.; Štefanić, P.P.; Letofsky-Papst, I.; Tkalec, M.; Balen, B. Toxicity of silver ions and differently coated silver nanoparticles in Allium cepa roots. Ecotoxicol. Environ. Saf. 2017, 137, 18–28. [Google Scholar] [CrossRef]
- Kaveh, R.; Li, Y.-S.; Ranjbar, S.; Tehrani, R.; Brueck, C.L.; Van Aken, B. Changes in Arabidopsis thaliana gene expression in response to silver nanoparticles and silver ions. Environ. Sci. Technol. 2013, 47, 10637–10644. [Google Scholar] [CrossRef]
- Dimkpa, C.O.; McLean, J.E.; Martineau, N.; Britt, D.W.; Haverkamp, R.; Anderson, A.J. Silver nanoparticles disrupt wheat (Triticum aestivum L.) growth in a sand matrix. Environ. Sci. Technol. 2013, 47, 1082–1090. [Google Scholar] [CrossRef] [PubMed]
- Qian, H.; Peng, X.; Han, X.; Ren, J.; Sun, L.; Fu, Z. Comparison of the toxicity of silver nanoparticles and silver ions on the growth of terrestrial plant model Arabidopsis thaliana. J. Environ. Sci. 2013, 25, 1947–1956. [Google Scholar] [CrossRef] [PubMed]
- Amooaghaie, R.; Tabatabaei, F.; Ahadi, A.-M. Role of hematin and sodium nitroprusside in regulating Brassica nigra seed germination under nanosilver and silver nitrate stresses. Ecotoxicol. Environ. Saf. 2015, 113, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Gubbins, E.J.; Batty, L.C.; Lead, J.R. Phytotoxicity of silver nanoparticles to Lemna minor L. Environ. Pollut. 2011, 159, 1551–1559. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Cheng, Y.; Espinasse, B.; Colman, B.P.; Auffan, M.; Wiesner, M.; Rose, J.; Liu, J.; Bernhardt, E.S. More than the ions: The effects of silver nanoparticles on Lolium multiflorum. Environ. Sci. Technol. 2011, 45, 2360–2367. [Google Scholar] [CrossRef] [PubMed]
- Ejaz, M.; Raja, N.I.; Mashwani, Z.U.R.; Ahmad, M.S.; Hussain, M.; Iqbal, M. Effect of silver nanoparticles and silver nitrate on growth of rice under biotic stress. IET Nanobiotechnol. 2018, 12, 927–932. [Google Scholar] [CrossRef]
- Yang, J.; Jiang, F.; Ma, C.; Rui, Y.; Rui, M.; Adeel, M.; Cao, W.; Xing, B. Alteration of crop yield and quality of wheat upon exposure to silver nanoparticles in a life cycle study. J. Agric. Food Chem. 2018, 66, 2589–2597. [Google Scholar] [CrossRef]
- Al-Huqail, A.A.; Hatata, M.M.; Al-Huqail, A.A.; Ibrahim, M.M. Preparation, characterization of silver phyto nanoparticles and their impact on growth potential of Lupinus termis L. seedlings. Saudi J. Biol. Sci. 2018, 25, 313–319. [Google Scholar] [CrossRef]
- Bakr, M.M.; Al-Ankily, M.M.; Shogaa, S.M.; Shamel, M. Attenuating Effect of Vitamin E against Silver Nano Particles Toxicity in Submandibular Salivary Glands. Bioengineering 2021, 8, 219. [Google Scholar] [CrossRef]










| Bacteria | Plants | Diameter of Inhibition Zone (mm) | MIC (µg/mL) | MBC (µg/mL) |
|---|---|---|---|---|
| E. coli | Coriander | 1.9 | 7.30 | 7.20 |
| Mint | 2.1 | 7.50 | 7.40 | |
| Aloe vera | 1.7 | 8.00 | 8.30 | |
| Lemongrass | 2.0 | 7.70 | 7.60 | |
| Control | 0.0 | - | - | |
| Staphylococcus aureus | Coriander | 1.8 | 7.20 | 7.10 |
| Mint | 1.7 | 7.90 | 7.80 | |
| Aloe vera | 1.6 | 7.80 | 7.70 | |
| Lemongrass | 1.9 | 7.40 | 7.50 | |
| Control | 0.0 | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmad, B.; Chang, L.; Satti, U.Q.; Rehman, S.u.; Arshad, H.; Mustafa, G.; Shaukat, U.; Wang, F.; Tong, C. Phyto-Synthesis, Characterization, and In Vitro Antibacterial Activity of Silver Nanoparticles Using Various Plant Extracts. Bioengineering 2022, 9, 779. https://doi.org/10.3390/bioengineering9120779
Ahmad B, Chang L, Satti UQ, Rehman Su, Arshad H, Mustafa G, Shaukat U, Wang F, Tong C. Phyto-Synthesis, Characterization, and In Vitro Antibacterial Activity of Silver Nanoparticles Using Various Plant Extracts. Bioengineering. 2022; 9(12):779. https://doi.org/10.3390/bioengineering9120779
Chicago/Turabian StyleAhmad, Bilal, Li Chang, Usama Qamar Satti, Sami ur Rehman, Huma Arshad, Ghazala Mustafa, Uzma Shaukat, Fenghua Wang, and Chunyi Tong. 2022. "Phyto-Synthesis, Characterization, and In Vitro Antibacterial Activity of Silver Nanoparticles Using Various Plant Extracts" Bioengineering 9, no. 12: 779. https://doi.org/10.3390/bioengineering9120779