Comparison of the Biomechanical Properties between Healthy and Whole Human and Porcine Stomachs
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection and Dissection
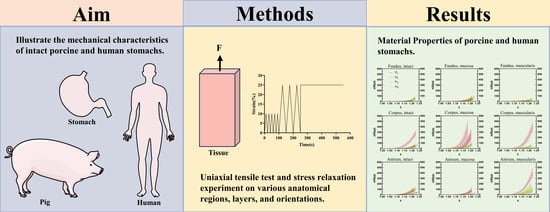
2.2. Mechanical Experiments
2.3. Mechanical Data Analysis
2.4. Statistical Analysis
3. Results
3.1. Morphological Data
3.2. Hyperelastic Mechanical Properties
3.3. Viscoelastic Mechanical Properties
4. Discussion
4.1. Hyperelastic Mechanical Properties
4.2. Viscoelastic Mechanical Properties
4.3. Anisotropic Mechanical Behavior
4.4. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Cancer statistics for the year 2020: An overview. Int. J. Cancer 2021, 149, 778–789. [Google Scholar] [CrossRef] [PubMed]
- López, M.J.; Carbajal, J.; Alfaro, A.L.; Saravia, L.G.; Zanabria, D.; Araujo, J.M.; Quispe, L.; Zevallos, A.; Buleje, J.L.; Cho, C.E.; et al. Characteristics of gastric cancer around the world. Crit. Rev. Oncol./Hematol. 2023, 181, 103841. [Google Scholar] [CrossRef] [PubMed]
- Van Cutsem, E.; Sagaert, X.; Topal, B.; Haustermans, K.; Prenen, H. Gastric cancer. Lancet 2016, 388, 2654–2664. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.S.; Badgwell, B.D. Current treatment and recent progress in gastric cancer. CA Cancer J. Clin. 2021, 71, 264–279. [Google Scholar] [CrossRef] [PubMed]
- Li, S.S.; Costantino, C.L.; Mullen, J.T. Morbidity and Mortality of Total Gastrectomy: A Comprehensive Analysis of 90-Day Outcomes. J. Gastrointest. Surg. 2019, 23, 1340–1348. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Liao, G.Q.; Liu, H.L.; Liu, S.; Tang, J. Meta-analysis of laparoscopy-assisted distal gastrectomy with D2 lymph node dissection for gastric cancer. J. Surg. Oncol. 2012, 105, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Best, L.M.; Mughal, M.; Gurusamy, K.S. Laparoscopic versus open gastrectomy for gastric cancer. Cochrane Database Syst. Rev. 2016, 3, Cd011389. [Google Scholar] [CrossRef]
- Bos, J.; Doornebosch, E.W.; Engbers, J.G.; Nyhuis, O.; Dodou, D. Methods for reducing peak pressure in laparoscopic grasping. Proc. Inst. Mech. Eng. H 2013, 227, 1292–1300. [Google Scholar] [CrossRef]
- Vogt, C.D.; Panoskaltsis-Mortari, A. Tissue engineering of the gastroesophageal junction. J. Tissue Eng. Regen. Med. 2020, 14, 855–868. [Google Scholar] [CrossRef]
- Ahluwalia, N.K.; Thompson, D.G.; Barlow, J.; Troncon, L.E.; Hollis, S. Relaxation responses of the human proximal stomach to distension during fasting and after food. Am. J. Physiol. 1994, 267, G166–G172. [Google Scholar] [CrossRef] [PubMed]
- Gregersen, H.; Gilja, O.H.; Hausken, T.; Heimdal, A.; Gao, C.; Matre, K.; Ødegaard, S.; Berstad, A. Mechanical properties in the human gastric antrum using B-mode ultrasonography and antral distension. Am. J. Physiol. Gastrointest. Liver Physiol. 2002, 283, G368–G375. [Google Scholar] [CrossRef] [PubMed]
- Carniel, E.L.; Albanese, A.; Fontanella, C.G.; Pavan, P.G.; Prevedello, L.; Salmaso, C.; Todros, S.; Toniolo, I.; Foletto, M. Biomechanics of stomach tissues and structure in patients with obesity. J. Mech. Behav. Biomed. Mater. 2020, 110, 103883. [Google Scholar] [CrossRef] [PubMed]
- Penning, C.; Vu, M.K.; Delemarre, J.B.V.M.; Masclee, A.A.M. Proximal gastric motor and sensory function in slow transit constipation. Scand. J. Gastroenterol. 2001, 36, 1267–1273. [Google Scholar] [CrossRef]
- Egorov, V.I.; Schastlivtsev, I.V.; Prut, E.V.; Baranov, A.O.; Turusov, R.A. Mechanical properties of the human gastrointestinal tract. J. Biomech. 2002, 35, 1417–1425. [Google Scholar] [CrossRef]
- Jia, Z.G.; Li, W.; Zhou, Z.R. Mechanical characterization of stomach tissue under uniaxial tensile action. J. Biomech. 2015, 48, 651–658. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Liao, D.; Gregersen, H. Tension and stress in the rat and rabbit stomach are location- and direction-dependent. Neurogastroenterol. Motil. 2005, 17, 388–398. [Google Scholar] [CrossRef]
- Tomalka, A.; Borsdorf, M.; Bol, M.; Siebert, T. Porcine Stomach Smooth Muscle Force Depends on History-Effects. Front. Physiol. 2017, 8, 802. [Google Scholar] [CrossRef]
- Zhao, J.; Liao, D.; Chen, P.; Kunwald, P.; Gregersen, H. Stomach stress and strain depend on location, direction and the layered structure. J. Biomech. 2008, 41, 3441–3447. [Google Scholar] [CrossRef]
- Rotta, G.; Kobiela, J.; Grymek, S.; Karczewska, M. Mechanical properties of the human stomach under uniaxial stress action. Curr. Sci. 2019, 116, 1886–1893. [Google Scholar] [CrossRef]
- Rosen, J.; Brown, J.D.; De, S.; Sinanan, M.; Hannaford, B. Biomechanical properties of abdominal organs in vivo and postmortem under compression loads. J. Biomech. Eng. 2008, 130, 021020. [Google Scholar] [CrossRef]
- Friis, S.J.; Hansen, T.S.; Poulsen, M.; Gregersen, H.; Brüel, A.; Vinge Nygaard, J. Biomechanical properties of the stomach: A comprehensive comparative analysis of human and porcine gastric tissue. J. Mech. Behav. Biomed. Mater. 2023, 138, 105614. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, J.; Liu, C.; Du, B.; Xiao, J.; Qian, L.; Zhang, Q.; Li, J. Development of a continuously perfused ex vivo kidney training model for laparoscopic partial nephrectomy: Validity and efficiency. Int. J. Surg. 2023, 109, 3919–3928. [Google Scholar] [CrossRef] [PubMed]
- Bauer, M.; Morales-Orcajo, E.; Klemm, L.; Seydewitz, R.; Fiebach, V.; Siebert, T.; Bol, M. Biomechanical and microstructural characterisation of the porcine stomach wall: Location- and layer-dependent investigations. Acta Biomater. 2020, 102, 83–99. [Google Scholar] [CrossRef] [PubMed]
- Aydin, R.C.; Brandstaeter, S.; Braeu, F.A.; Steigenberger, M.; Marcus, R.P.; Nikolaou, K.; Notohamiprodjo, M.; Cyron, C.J. Experimental characterization of the biaxial mechanical properties of porcine gastric tissue. J. Mech. Behav. Biomed. Mater. 2017, 74, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Brouwer, I.; Ustin, J.; Bentley, L.; Sherman, A.; Dhruv, N.; Tendick, F. Measuring in vivo animal soft tissue properties for haptic modeling in surgical simulation. Stud. Health Technol. Inf. 2001, 81, 69–74. [Google Scholar]
- Lee, A.H.; Elliott, D.M. Freezing does not alter multiscale tendon mechanics and damage mechanisms in tension. Ann. N. Y. Acad. Sci. 2017, 1409, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Takaza, M.; Moerman, K.M.; Gindre, J.; Lyons, G.; Simms, C.K. The anisotropic mechanical behaviour of passive skeletal muscle tissue subjected to large tensile strain. J. Mech. Behav. Biomed. Mater. 2013, 17, 209–220. [Google Scholar] [CrossRef] [PubMed]
- Hobson, A.R.; Aziz, Q. Oesophagus and stomach motility. Medicine 2011, 39, 210–213. [Google Scholar] [CrossRef]
- Mahadevan, V. Anatomy of the stomach. Surgery 2017, 35, 608–611. [Google Scholar] [CrossRef]
- Hightower, N.C.; Keeton, W.T.; Dworken, H.J.; Sircus, W. Human digestive system. Encyclopedia Britannica, 4 November 2020. [Google Scholar]
- Singh, G.; Chanda, A. Mechanical properties of whole-body soft human tissues: A review. Biomed. Mater. 2021, 16, 062004. [Google Scholar] [CrossRef] [PubMed]







| N | Sex | Age (Years) | Weight (Kg) | Height (m) | BMI (Kg/m2) |
|---|---|---|---|---|---|
| 1 | F | 50 | 48 | 1.61 | 18.52 |
| 2 | M | 40 | 79 | 1.83 | 23.59 |
| 3 | F | 43 | 61 | 1.63 | 22.96 |
| 4 | M | 63 | 75 | 1.78 | 23.67 |
| 5 | M | 55 | 67 | 1.70 | 23.18 |
| 6 | M | 58 | 60 | 1.65 | 22.04 |
| 7 | M | 71 | 75 | 1.74 | 24.77 |
| 8 | M | 31 | 74 | 1.77 | 23.62 |
| 9 | M | 36 | 63 | 1.80 | 19.44 |
| Mean ± SD | 50 ± 12 | 67 ± 9 | 1.72 ± 0.07 | 22.42 ± 1.97 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, F.; Liu, J.; Liu, X.; Wu, Y.; Qian, L.; Huang, W.; Li, Y. Comparison of the Biomechanical Properties between Healthy and Whole Human and Porcine Stomachs. Bioengineering 2024, 11, 233. https://doi.org/10.3390/bioengineering11030233
Li F, Liu J, Liu X, Wu Y, Qian L, Huang W, Li Y. Comparison of the Biomechanical Properties between Healthy and Whole Human and Porcine Stomachs. Bioengineering. 2024; 11(3):233. https://doi.org/10.3390/bioengineering11030233
Chicago/Turabian StyleLi, Feifei, Jiannan Liu, Xiaoyun Liu, Yaobin Wu, Lei Qian, Wenhua Huang, and Yanbing Li. 2024. "Comparison of the Biomechanical Properties between Healthy and Whole Human and Porcine Stomachs" Bioengineering 11, no. 3: 233. https://doi.org/10.3390/bioengineering11030233