Aqueous Rechargeable Sodium-Ion Batteries: From Liquid to Hydrogel
Abstract
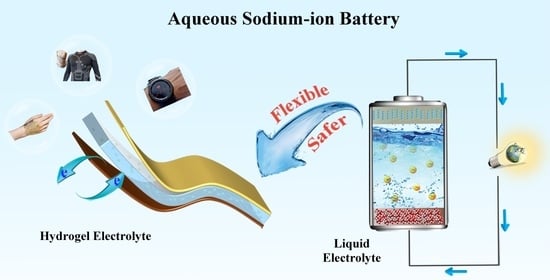
:1. Introduction
2. Electrolytes in ASIBs
2.1. Liquid Electrolyte

2.2. Hydrogel Electrolyte
3. Electrode Materials
3.1. Cathode Materials
3.1.1. Polyanionic Compound
3.1.2. Manganese Oxide

3.1.3. Prussian Blue Analogues
3.2. Anode Materials
3.2.1. NASICON Structure
3.2.2. Organic Materials
3.2.3. Prussian Blue and Analogues
3.2.4. Other Materials
4. Future Applications

5. Summary and Prospects
- (1)
- There is still a significant challenge to overcome with regard to the narrow electrochemical stability window of aqueous electrolytes that impacts their cyclic stability. Research in this area should be conducted and developed further.
- (2)
- Future research and exploration of sodium-ion batteries with hydrogel electrolytes should be intensified in order to develop flexible devices and micro-devices that are well suited to the Internet of Things.
- (3)
- The decomposition of electrode materials and reactions with oxygen or water in aqueous electrolytes will decrease the chemical stability of electrode materials and further reduce the stability of sodium-ion batteries in aqueous systems. Efforts should be made to conduct relevant research and strengthen it further.
- (4)
- Electrolyte/electrode surface optimizations hold great promise in regulating the formation and evolution of interphases, the in-depth understanding of which will assist in rationally optimizing electrode/electrolyte compatibility.
- (5)
- The method of high-throughput computing can help accelerate research and development in this area by enabling the development of theoretical models that facilitate rapid screening and prediction of electrode materials and electrolytes.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pan, H.; Hu, Y.-S.; Chen, L. Room-temperature stationary sodium-ion batteries for large-scale electric energy storage. Energy Environ. Sci. 2013, 6, 2338–2360. [Google Scholar] [CrossRef]
- Yabuuchi, N.; Kubota, K.; Dahbi, M.; Komaba, S. Research development on sodium-ion batteries. Chem. Rev. 2014, 114, 11636–11682. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Li, X.; Xu, Y.; Chen, J.; Lv, M.; Yang, M.; Chen, W. Understanding the Accelerated Sodium-Ion-Transport Mechanism of an Interfacial Modified Polyacrylonitrile Separator. J. Phys. Chem. C 2022, 126, 8238–8247. [Google Scholar] [CrossRef]
- Song, K.; Liu, J.; Dai, H.; Zhao, Y.; Sun, S.; Zhang, J.; Qin, C.; Yan, P.; Guo, F.; Wang, C. Atomically dispersed Ni induced by ultrahigh N-doped carbon enables stable sodium storage. Chem 2021, 7, 2684–2694. [Google Scholar] [CrossRef]
- Wan, Y.; Song, K.; Chen, W.; Qin, C.; Zhang, X.; Zhang, J.; Dai, H.; Hu, Z.; Yan, P.; Liu, C. Ultra-High Initial Coulombic Efficiency Induced by Interface Engineering Enables Rapid, Stable Sodium Storage. Angew. Chem. 2021, 133, 11582–11587. [Google Scholar] [CrossRef]
- Chen, X.; Fang, Y.; Lu, H.; Li, H.; Feng, X.; Chen, W.; Ai, X.; Yang, H.; Cao, Y. Microstructure-dependent charge/discharge behaviors of hollow carbon spheres and its implication for sodium storage mechanism on hard carbon anodes. Small 2021, 17, 2102248. [Google Scholar] [CrossRef]
- Zhao, A.; Yuan, T.; Li, P.; Liu, C.; Cong, H.; Pu, X.; Chen, Z.; Ai, X.; Yang, H.; Cao, Y. A novel Fe-defect induced pure-phase Na4Fe2.91(PO4)2P2O7 cathode material with high capacity and ultra-long lifetime for low-cost sodium-ion batteries. Nano Energy 2022, 91, 106680. [Google Scholar] [CrossRef]
- Fedoseeva, Y.V.; Shlyakhova, E.V.; Stolyarova, S.G.; Vorfolomeeva, A.A.; Grebenkina, M.A.; Makarova, A.A.; Shubin, Y.V.; Okotrub, A.V.; Bulusheva, L.G. Brominated Porous Nitrogen-Doped Carbon Materials for Sodium-Ion Storage. Batteries 2022, 8, 114. [Google Scholar] [CrossRef]
- Chao, D.; Zhou, W.; Xie, F.; Ye, C.; Li, H.; Jaroniec, M.; Qiao, S.-Z. Roadmap for advanced aqueous batteries: From design of materials to applications. Sci. Adv. 2020, 6, eaba4098. [Google Scholar] [CrossRef]
- Cano, Z.P.; Banham, D.; Ye, S.; Hintennach, A.; Lu, J.; Fowler, M.; Chen, Z. Batteries and fuel cells for emerging electric vehicle markets. Nat. Energy 2018, 3, 279–289. [Google Scholar] [CrossRef]
- Liu, Z.; Huang, Y.; Huang, Y.; Yang, Q.; Li, X.; Huang, Z.; Zhi, C. Voltage issue of aqueous rechargeable metal-ion batteries. Chem. Soc. Rev. 2020, 49, 180–232. [Google Scholar] [CrossRef] [PubMed]
- Bin, D.; Wang, F.; Tamirat, A.G.; Suo, L.; Wang, Y.; Wang, C.; Xia, Y. Progress in aqueous rechargeable sodium-ion batteries. Adv. Energy Mater. 2018, 8, 1703008. [Google Scholar] [CrossRef]
- Guo, Z.; Zhao, Y.; Ding, Y.; Dong, X.; Chen, L.; Cao, J.; Wang, C.; Xia, Y.; Peng, H.; Wang, Y. Multi-functional flexible aqueous sodium-ion batteries with high safety. Chem 2017, 3, 348–362. [Google Scholar] [CrossRef]
- Qiu, S.; Xu, Y.; Wu, X.; Ji, X. Prussian blue analogues as electrodes for aqueous monovalent ion batteries. Electrochem. Energy Rev. 2022, 5, 242–262. [Google Scholar] [CrossRef]
- Li, W.; Dahn, J.R.; Wainwright, D.S. Rechargeable lithium batteries with aqueous electrolytes. Science 1994, 264, 1115–1118. [Google Scholar] [CrossRef]
- Jin, T.; Ji, X.; Wang, P.F.; Zhu, K.; Zhang, J.; Cao, L.; Chen, L.; Cui, C.; Deng, T.; Liu, S. High-Energy Aqueous Sodium-Ion Batteries. Angew. Chem. 2021, 133, 12050–12055. [Google Scholar] [CrossRef]
- Liu, M.; Ao, H.; Jin, Y.; Hou, Z.; Zhang, X.; Zhu, Y.; Qian, Y. Aqueous rechargeable sodium ion batteries: Developments and prospects. Mater. Today Energy 2020, 17, 100432. [Google Scholar] [CrossRef]
- You, Y.; Sang, Z.; Liu, J. Recent developments on aqueous sodium-ion batteries. Mater. Technol. 2016, 31, 501–509. [Google Scholar] [CrossRef]
- Boyd, S.; Augustyn, V. Transition metal oxides for aqueous sodium-ion electrochemical energy storage. Inorg. Chem. Front. 2018, 5, 999–1015. [Google Scholar] [CrossRef]
- Larcher, D.; Tarascon, J.-M. Towards greener and more sustainable batteries for electrical energy storage. Nat. Chem. 2015, 7, 19–29. [Google Scholar] [CrossRef]
- Dunn, B.; Kamath, H.; Tarascon, J.-M. Electrical energy storage for the grid: A battery of choices. Science 2011, 334, 928–935. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zhang, X.; Mi, L.; Liu, C.; Zhang, J.; Cui, S.; Feng, X.; Cao, Y.; Shen, C. High-performance flexible freestanding anode with hierarchical 3D carbon-networks/Fe7S8/graphene for applicable sodium-ion batteries. Adv. Mater. 2019, 31, 1806664. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Jing, X.; Jiang, K.; Wang, D. Observation of structural decomposition of Na3V2(PO4)3 and Na3V2(PO4)2F3 as cathodes for aqueous zn-ion batteries. ACS Appl. Energy Mater. 2021, 4, 2797–2807. [Google Scholar] [CrossRef]
- Zhang, Q.; Liao, C.; Zhai, T.; Li, H. A high rate 1.2 V aqueous sodium-ion battery based on all NASICON structured NaTi2(PO4)3 and Na3V2(PO4)3. Electrochim. Acta 2016, 196, 470–478. [Google Scholar] [CrossRef]
- Kumar, P.R.; Jung, Y.H.; Lim, C.H.; Kim, D.K. Na3V2O2x(PO4)2F3−2x: A stable and high-voltage cathode material for aqueous sodium-ion batteries with high energy density. J. Mater. Chem. A 2015, 3, 6271–6275. [Google Scholar] [CrossRef]
- Demir-Cakan, R.; Palacin, M.R.; Croguennec, L. Rechargeable aqueous electrolyte batteries: From univalent to multivalent cation chemistry. J. Mater. Chem. A 2019, 7, 20519–20539. [Google Scholar] [CrossRef]
- Huang, J.; Guo, Z.; Ma, Y.; Bin, D.; Wang, Y.; Xia, Y. Recent progress of rechargeable batteries using mild aqueous electrolytes. Small Methods 2019, 3, 1800272. [Google Scholar] [CrossRef]
- Kim, H.; Hong, J.; Park, K.-Y.; Kim, H.; Kim, S.-W.; Kang, K. Aqueous rechargeable Li and Na ion batteries. Chem. Rev. 2014, 114, 11788–11827. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, T.; Chen, C.; Ling, M.; Lin, Z.; Zhang, S.; Pan, F.; Liang, C. High-performance aqueous symmetric sodium-ion battery using NASICON-structured Na2VTi(PO4)3. Nano Res. 2018, 11, 490–498. [Google Scholar] [CrossRef]
- Park, S.I.; Gocheva, I.; Okada, S.; Yamaki, J.-I. Electrochemical properties of NaTi2(PO4)3 anode for rechargeable aqueous sodium-ion batteries. J. Electrochem. Soc. 2011, 158, A1067. [Google Scholar] [CrossRef]
- Qin, H.; Song, Z.; Zhan, H.; Zhou, Y. Aqueous rechargeable alkali-ion batteries with polyimide anode. J. Power Sources 2014, 249, 367–372. [Google Scholar] [CrossRef]
- Zhang, H.; Jeong, S.; Qin, B.; Vieira Carvalho, D.; Buchholz, D.; Passerini, S. Towards High-Performance Aqueous Sodium-Ion Batteries: Stabilizing the Solid/Liquid Interface for NASICON-Type Na2VTi(PO4)3 using Concentrated Electrolytes. ChemSusChem 2018, 11, 1382–1389. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.; Lei, P.; Huang, Y.; Tian, G.; Xiang, X. Improved electrochemical performance of graphene-integrated NaTi2(PO4)3/C anode in high-concentration electrolyte for aqueous sodium-ion batteries. J. Electroanal. Chem. 2019, 838, 66–72. [Google Scholar] [CrossRef]
- Nakamoto, K.; Sakamoto, R.; Ito, M.; Kitajou, A.; Okada, S. Effect of concentrated electrolyte on aqueous sodium-ion battery with sodium manganese hexacyanoferrate cathode. Electrochemistry 2017, 85, 179–185. [Google Scholar] [CrossRef]
- Han, J.; Zarrabeitia, M.; Mariani, A.; Jusys, Z.; Hekmatfar, M.; Zhang, H.; Geiger, D.; Kaiser, U.; Behm, R.J.; Varzi, A. Halide-free water-in-salt electrolytes for stable aqueous sodium-ion batteries. Nano Energy 2020, 77, 105176. [Google Scholar] [CrossRef]
- Chua, R.; Cai, Y.; Lim, P.Q.; Kumar, S.; Satish, R.; Manalastas Jr, W.; Ren, H.; Verma, V.; Meng, S.; Morris, S.A. Hydrogen-Bonding Interactions in Hybrid Aqueous/Nonaqueous Electrolytes Enable Low-Cost and Long-Lifespan Sodium-Ion Storage. ACS Appl. Mater. Interfaces 2020, 12, 22862–22872. [Google Scholar] [CrossRef]
- Wang, J.; Huang, Y.; Liu, B.; Li, Z.; Zhang, J.; Yang, G.; Hiralal, P.; Jin, S.; Zhou, H. Flexible and anti-freezing zinc-ion batteries using a guar-gum/sodium-alginate/ethylene-glycol hydrogel electrolyte. Energy Storage Mater. 2021, 41, 599–605. [Google Scholar] [CrossRef]
- Li, J.; Yu, P.; Zhang, S.; Wen, Z.; Wen, Y.; Zhou, W.; Dong, X.; Liu, Y.; Liang, Y. Mild synthesis of superadhesive hydrogel electrolyte with low interfacial resistance and enhanced ionic conductivity for flexible zinc ion battery. J. Colloid Interface Sci. 2021, 600, 586–593. [Google Scholar] [CrossRef]
- Liu, Z.; Liang, G.; Zhan, Y.; Li, H.; Wang, Z.; Ma, L.; Wang, Y.; Niu, X.; Zhi, C. A soft yet device-level dynamically super-tough supercapacitor enabled by an energy-dissipative dual-crosslinked hydrogel electrolyte. Nano Energy 2019, 58, 732–742. [Google Scholar] [CrossRef]
- Chen, C.R.; Qin, H.; Cong, H.P.; Yu, S.H. A highly stretchable and real-time healable supercapacitor. Adv. Mater. 2019, 31, 1900573. [Google Scholar] [CrossRef]
- Li, Y.; Wang, X.; Fu, Y.-n.; Wei, Y.; Zhao, L.; Tao, L. Self-adapting hydrogel to improve the therapeutic effect in wound-healing. ACS Appl. Mater. Interfaces 2018, 10, 26046–26055. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Chen, Y.; Rehman, H.U.; Chen, Z.; Yang, Z.; Wang, M.; Li, H.; Liu, H. Ultratough, self-healing, and tissue-adhesive hydrogel for wound dressing. ACS Appl. Mater. Interfaces 2018, 10, 33523–33531. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, J.; Chen, S.; Lei, T.; Kim, Y.; Niu, S.; Wang, H.; Wang, X.; Foudeh, A.M.; Tok, J.B.-H. Soft and elastic hydrogel-based microelectronics for localized low-voltage neuromodulation. Nat. Biomed. Eng. 2019, 3, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Gai, J.; Song, K.; Chen, W. Advances in electrode/electrolyte interphase for sodium-ion batteries from half cells to full cells. Cell Rep. Phys. Sci. 2022, 3, 100868. [Google Scholar] [CrossRef]
- Zhang, J.; Meng, Z.; Yang, D.; Song, K.; Mi, L.; Zhai, Y.; Guan, X.; Chen, W. Enhanced interfacial compatibility of FeS@N, SC anode with ester-based electrolyte enables stable sodium-ion full cells. J. Energy Chem. 2022, 68, 27–34. [Google Scholar] [CrossRef]
- Li, G.; Lou, X.; Peng, C.; Liu, C.; Chen, W. Interface chemistry for sodium metal anodes/batteries: A review. Chem. Synth. 2022, 2, 16. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, B.; Xiao, S.; Liu, L.; Wen, Z.; Wu, Y. A nanocomposite of MoO3 coated with PPy as an anode material for aqueous sodium rechargeable batteries with excellent electrochemical performance. Electrochim. Acta 2014, 116, 512–517. [Google Scholar] [CrossRef]
- Wu, X.; Sun, M.; Shen, Y.; Qian, J.; Cao, Y.; Ai, X.; Yang, H. Energetic Aqueous Rechargeable Sodium-Ion Battery Based on Na2CuFe(CN)6–NaTi2(PO4)3 Intercalation Chemistry. ChemSusChem 2014, 7, 407–411. [Google Scholar] [CrossRef]
- Wang, Y.; Feng, Z.; Laul, D.; Zhu, W.; Provencher, M.; Trudeau, M.L.; Guerfi, A.; Zaghib, K. Ultra-low cost and highly stable hydrated FePO4 anodes for aqueous sodium-ion battery. J. Power Sources 2018, 374, 211–216. [Google Scholar] [CrossRef]
- Ke, L.; Dong, J.; Lin, B.; Yu, T.; Wang, H.; Zhang, S.; Deng, C. A NaV3(PO4)3@C hierarchical nanofiber in high alignment: Exploring a novel high-performance anode for aqueous rechargeable sodium batteries. Nanoscale 2017, 9, 4183–4190. [Google Scholar] [CrossRef]
- Hung, T.-F.; Lan, W.-H.; Yeh, Y.-W.; Chang, W.-S.; Yang, C.-C.; Lin, J.-C. Hydrothermal synthesis of sodium titanium phosphate nanoparticles as efficient anode materials for aqueous sodium-ion batteries. ACS Sustain. Chem. Eng. 2016, 4, 7074–7079. [Google Scholar] [CrossRef]
- Sharma, L.; Nakamoto, K.; Sakamoto, R.; Okada, S.; Barpanda, P. Na2FePO4F Fluorophosphate as Positive Insertion Material for Aqueous Sodium-Ion Batteries. ChemElectroChem 2019, 6, 444–449. [Google Scholar] [CrossRef]
- Gao, H.; Goodenough, J.B. An aqueous symmetric sodium-ion battery with NASICON-structured Na3MnTi(PO4)3. Angew. Chem. 2016, 128, 12960–12964. [Google Scholar] [CrossRef]
- Shan, X.; Charles, D.S.; Lei, Y.; Qiao, R.; Wang, G.; Yang, W.; Feygenson, M.; Su, D.; Teng, X. Bivalence Mn5O8 with hydroxylated interphase for high-voltage aqueous sodium-ion storage. Nat. Commun. 2016, 7, 13370. [Google Scholar] [CrossRef] [PubMed]
- Nakamoto, K.; Kano, Y.; Kitajou, A.; Okada, S. Electrolyte dependence of the performance of a Na2FeP2O7//NaTi2(PO4)3 rechargeable aqueous sodium-ion battery. J. Power Sources 2016, 327, 327–332. [Google Scholar] [CrossRef]
- Yang, X.; Zhou, J.; Huo, T.; Lv, Y.; Pan, J.; Chen, L.; Tang, X.; Zhao, Y.; Liu, H.; Gao, Q. Metabolic insights into the enhanced nitrogen removal of anammox by montmorillonite at reduced temperature. Chem. Eng. J. 2021, 410, 128290. [Google Scholar] [CrossRef]
- Zhang, H.; Qin, B.; Han, J.; Passerini, S. Aqueous/nonaqueous hybrid electrolyte for sodium-ion batteries. ACS Energy Lett. 2018, 3, 1769–1770. [Google Scholar] [CrossRef]
- Kumar, P.R.; Kheireddine, A.; Nisar, U.; Shakoor, R.; Essehli, R.; Amin, R.; Belharouak, I. Na4MnV(PO4)3-rGO as Advanced cathode for aqueous and non-aqueous sodium ion batteries. J. Power Sources 2019, 429, 149–155. [Google Scholar] [CrossRef]
- Qiu, S.; Lucero, M.; Wu, X.; Wang, Q.; Wang, M.; Wang, Y.; Samarakoon, W.S.; Bolding, M.R.; Yang, Z.; Huang, Y. Revealing the Fast and Durable Na+ Insertion Reactions in a Layered Na3Fe3(PO4)4 Anode for Aqueous Na-Ion Batteries. ACS Mater. Au 2021, 2, 63–71. [Google Scholar] [CrossRef]
- Nakamoto, K.; Sakamoto, R.; Sawada, Y.; Ito, M.; Okada, S. Over 2V aqueous sodium-ion battery with Prussian blue-type electrodes. Small Methods 2019, 3, 1800220. [Google Scholar] [CrossRef]
- Liu, S.; Wang, L.; Liu, J.; Zhou, M.; Nian, Q.; Feng, Y.; Tao, Z.; Shao, L. Na3V2(PO4)2F3–SWCNT: A high voltage cathode for non-aqueous and aqueous sodium-ion batteries. J. Mater. Chem. A 2019, 7, 248–256. [Google Scholar] [CrossRef]
- Chen, J.; Liu, C.; Yu, Z.; Qu, J.; Wang, C.; Lai, L.; Wei, L.; Chen, Y. High-energy-density aqueous sodium-ion batteries enabled by chromium hexacycnochromate anodes. Chem. Eng. J. 2021, 415, 129003. [Google Scholar] [CrossRef]
- Tang, W.; Song, X.; Du, Y.; Peng, C.; Lin, M.; Xi, S.; Tian, B.; Zheng, J.; Wu, Y.; Pan, F. High-performance NaFePO4 formed by aqueous ion-exchange and its mechanism for advanced sodium ion batteries. J. Mater. Chem. A 2016, 4, 4882–4892. [Google Scholar] [CrossRef]
- Oh, S.-M.; Myung, S.-T.; Hassoun, J.; Scrosati, B.; Sun, Y.-K. Reversible NaFePO4 electrode for sodium secondary batteries. Electrochem. Commun. 2012, 22, 149–152. [Google Scholar] [CrossRef]
- Vujković, M.; Mentus, S. Fast sodiation/desodiation reactions of electrochemically delithiated olivine LiFePO4 in aerated aqueous NaNO3 solution. J. Power Sources 2014, 247, 184–188. [Google Scholar] [CrossRef]
- Zhou, W.; Xue, L.; Lü, X.; Gao, H.; Li, Y.; Xin, S.; Fu, G.; Cui, Z.; Zhu, Y.; Goodenough, J.B. NaxMV(PO4)3(M = Mn, Fe, Ni) structure and properties for sodium extraction. Nano Lett. 2016, 16, 7836–7841. [Google Scholar] [CrossRef] [PubMed]
- Ren, M.; Fang, H.; Wang, C.; Li, H.; Li, F. Advances on manganese-oxide-based cathodes for Na-ion batteries. Energy Fuels 2020, 34, 13412–13426. [Google Scholar] [CrossRef]
- Guo, X.; Wang, Z.; Deng, Z.; Wang, B.; Chen, X.; Ong, S.P. Design principles for aqueous Na-ion battery cathodes. Chem. Mater. 2020, 32, 6875–6885. [Google Scholar] [CrossRef]
- Tevar, A.D.; De Graef, M.; Whitacre, J. Cycling-Induced Crystallographic and Morphological Changes in Na4Mn9O18; ECS Meeting Abstracts; IOP Publishing: Bristol, UK, 2008; p. 642. [Google Scholar]
- Whitacre, J.; Tevar, A.; Sharma, S. Na4Mn9O18 as a positive electrode material for an aqueous electrolyte sodium-ion energy storage device. Electrochem. Commun. 2010, 12, 463–466. [Google Scholar] [CrossRef]
- Cao, Y.; Xiao, L.; Wang, W.; Choi, D.; Nie, Z.; Yu, J.; Saraf, L.V.; Yang, Z.; Liu, J. Reversible sodium ion insertion in single crystalline manganese oxide nanowires with long cycle life. Adv. Mater. 2011, 23, 3155–3160. [Google Scholar] [CrossRef]
- Chae, M.S.; Chakraborty, A.; Kunnikuruvan, S.; Attias, R.; Maddukuri, S.; Gofer, Y.; Major, D.T.; Aurbach, D. Vacancy-Driven High Rate Capabilities in Calcium-Doped Na0.4MnO2 Cathodes for Aqueous Sodium-Ion Batteries. Adv. Energy Mater. 2020, 10, 2002077. [Google Scholar] [CrossRef]
- Paolella, A.; Faure, C.; Timoshevskii, V.; Marras, S.; Bertoni, G.; Guerfi, A.; Vijh, A.; Armand, M.; Zaghib, K. A review on hexacyanoferrate-based materials for energy storage and smart windows: Challenges and perspectives. J. Mater. Chem. A 2017, 5, 18919–18932. [Google Scholar] [CrossRef]
- Guo, X.; Wang, Z.; Deng, Z.; Li, X.; Wang, B.; Chen, X.; Ong, S.P. Water contributes to higher energy density and cycling stability of Prussian blue analogue cathodes for aqueous sodium-ion batteries. Chem. Mater. 2019, 31, 5933–5942. [Google Scholar] [CrossRef]
- Ge, J.; Fan, L.; Rao, A.M.; Zhou, J.; Lu, B. Surface-substituted Prussian blue analogue cathode for sustainable potassium-ion batteries. Nat. Sustain. 2022, 5, 225–234. [Google Scholar] [CrossRef]
- Wessells, C.D.; Peddada, S.V.; Huggins, R.A.; Cui, Y. Nickel hexacyanoferrate nanoparticle electrodes for aqueous sodium and potassium ion batteries. Nano Lett. 2011, 11, 5421–5425. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Jiang, Y.; Liu, Y.; Ma, J.; Sun, T.; Zhu, N. High-stability monoclinic nickel hexacyanoferrate cathode materials for ultrafast aqueous sodium ion battery. Chem. Eng. J. 2020, 388, 124228. [Google Scholar] [CrossRef]
- Niu, L.; Chen, L.; Zhang, J.; Jiang, P.; Liu, Z. Revisiting the open-framework zinc hexacyanoferrate: The role of ternary electrolyte and sodium-ion intercalation mechanism. J. Power Sources 2018, 380, 135–141. [Google Scholar] [CrossRef]
- Li, W.; Zhang, F.; Xiang, X.; Zhang, X. Nickel-Substituted Copper Hexacyanoferrate as a Superior Cathode for Aqueous Sodium-Ion Batteries. ChemElectroChem 2018, 5, 350–354. [Google Scholar] [CrossRef]
- Fernández-Ropero, A.; Piernas-Muñoz, M.; Castillo-Martínez, E.; Rojo, T.; Casas-Cabanas, M. Electrochemical characterization of NaFe2 (CN) 6 Prussian blue as positive electrode for aqueous sodium-ion batteries. Electrochim. Acta 2016, 210, 352–357. [Google Scholar] [CrossRef]
- Zhao, F.; Wang, Y.; Xu, X.; Liu, Y.; Song, R.; Lu, G.; Li, Y. Cobalt hexacyanoferrate nanoparticles as a high-rate and ultra-stable supercapacitor electrode material. ACS Appl. Mater. Interfaces 2014, 6, 11007–11012. [Google Scholar] [CrossRef]
- Pasta, M.; Wang, R.Y.; Ruffo, R.; Qiao, R.; Lee, H.-W.; Shyam, B.; Guo, M.; Wang, Y.; Wray, L.A.; Yang, W. Manganese–cobalt hexacyanoferrate cathodes for sodium-ion batteries. J. Mater. Chem. A 2016, 4, 4211–4223. [Google Scholar] [CrossRef]
- Fernßndez-Ropero, A.; Saurel, D.; Acebedo, B.; Rojo, T.; Casas-Cabanas, M. Electrochemical characterization of NaFePO4 as positive electrode in aqueous sodium-ion batteries. J. Power Sources 2015, 291, 40–45. [Google Scholar] [CrossRef]
- Pang, G.; Yuan, C.; Nie, P.; Ding, B.; Zhu, J.; Zhang, X. Synthesis of NASICON-type structured NaTi2(PO4)3–graphene nanocomposite as an anode for aqueous rechargeable Na-ion batteries. Nanoscale 2014, 6, 6328–6334. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Liu, Q.; Chen, C.; Li, M.; Meng, X.; Bie, X.; Wei, Y.; Huang, Y.; Du, F.; Wang, C. NASICON-structured NaTi2(PO4)3@C nanocomposite as the low operation-voltage anode material for high-performance sodium-ion batteries. ACS Appl. Mater. Interfaces 2016, 8, 2238–2246. [Google Scholar] [CrossRef]
- Li, Z.; Young, D.; Xiang, K.; Carter, W.C.; Chiang, Y.M. Towards high power high energy aqueous sodium-ion batteries: The NaTi2(PO4)3/Na0.44MnO2 system. Adv. Energy Mater. 2013, 3, 290–294. [Google Scholar] [CrossRef]
- Rajagopalan, R.; Zhang, Z.; Tang, Y.; Jia, C.; Ji, X.; Wang, H. Understanding crystal structures, ion diffusion mechanisms and sodium storage behaviors of NASICON materials. Energy Stor. Mater. 2021, 34, 171–193. [Google Scholar] [CrossRef]
- He, B.; Yin, K.; Gong, W.; Xiong, Y.; Zhang, Q.; Yang, J.; Wang, Z.; Wang, Z.; Chen, M.; Man, P. NaTi2(PO4)3 hollow nanoparticles encapsulated in carbon nanofibers as novel anodes for flexible aqueous rechargeable sodium-ion batteries. Nano Energy 2021, 82, 105764. [Google Scholar] [CrossRef]
- Cho, B.; Lim, H.; Lee, H.-N.; Park, Y.M.; Kim, H.; Kim, H.-J. High-capacity and cycling-stable polypyrrole-coated MWCNT@ polyimide core-shell nanowire anode for aqueous rechargeable sodium-ion battery. Surf. Coat. Technol. 2021, 407, 126797. [Google Scholar] [CrossRef]
- Han, C.; Zhu, J.; Zhi, C.; Li, H. The rise of aqueous rechargeable batteries with organic electrode materials. J. Mater. Chem. A 2020, 8, 15479–15512. [Google Scholar] [CrossRef]
- Wei, Y.; Hu, Q.; Cao, Y.; Fang, D.; Xu, W.; Jiang, M.; Huang, J.; Liu, H.; Fan, X. Polypyrrole nanotube arrays on carbonized cotton textile for aqueous sodium battery. Org. Electron. 2017, 46, 211–217. [Google Scholar] [CrossRef]
- Wang, B.; Wang, X.; Liang, C.; Yan, M.; Jiang, Y. An All-Prussian-Blue-Based Aqueous Sodium-Ion Battery. ChemElectroChem 2019, 6, 4848–4853. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, D.; Niu, F.; Li, X.; Wang, C.; Yang, J. FeFe(CN)6 Nanocubes as a Bipolar Electrode Material in Aqueous Symmetric Sodium-Ion Batteries. ChemPlusChem 2017, 82, 1170–1173. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Huang, Q.; Wu, T.; Lu, L. Polyanion Sodium Vanadium Phosphate for Next Generation of Sodium-Ion Batteries—A Review. Adv. Funct. Mater. 2020, 30, 2001289. [Google Scholar] [CrossRef]
- Li, L.; Zhang, Y.; Lu, H.; Wang, Y.; Xu, J.; Zhu, J.; Zhang, C.; Liu, T. Cryopolymerization enables anisotropic polyaniline hybrid hydrogels with superelasticity and highly deformation-tolerant electrochemical energy storage. Nat. Commun. 2020, 11, 62. [Google Scholar] [CrossRef]
- Yun, T.G.; Park, M.; Kim, D.-H.; Kim, D.; Cheong, J.Y.; Bae, J.G.; Han, S.M.; Kim, I.-D. All-transparent stretchable electrochromic supercapacitor wearable patch device. Acs Nano 2019, 13, 3141–3150. [Google Scholar] [CrossRef]
- Gu, J.; Cui, K.; Niu, S.; Ge, Y.; Liu, Y.; Ma, Z.; Wang, C.; Li, X.; Wang, X. Smart configuration of cobalt hexacyanoferrate assembled on carbon fiber cloths for fast aqueous flexible sodium ion pseudocapacitor. J. Colloid Interface Sci. 2021, 594, 522–530. [Google Scholar] [CrossRef]
- Kang, J.; Tok, J.B.-H.; Bao, Z. Self-healing soft electronics. Nat. Electron. 2019, 2, 144–150. [Google Scholar] [CrossRef]
- Lopez, J.; Mackanic, D.G.; Cui, Y.; Bao, Z. Designing polymers for advanced battery chemistries. Nat. Rev. Mater. 2019, 4, 312–330. [Google Scholar] [CrossRef]
- Kutbee, A.T.; Bahabry, R.R.; Alamoudi, K.O.; Ghoneim, M.T.; Cordero, M.D.; Almuslem, A.S.; Gumus, A.; Diallo, E.M.; Nassar, J.M.; Hussain, A.M. Flexible and biocompatible high-performance solid-state micro-battery for implantable orthodontic system. NPJ Flex. Electron. 2017, 1, 7. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhu, K.; Li, J.; Li, Q.; Deng, B.; Zhang, P.; Wang, Q.; Guo, C.; Wang, W.; Liu, W. Leaf-Inspired Flexible Thermoelectric Generators with High Temperature Difference Utilization Ratio and Output Power in Ambient Air. Adv. Sci. 2021, 8, 2004947. [Google Scholar] [CrossRef]
- Zhao, C.-D.; Guo, J.-Z.; Gu, Z.-Y.; Wang, X.-T.; Zhao, X.-X.; Li, W.-H.; Yu, H.-Y.; Wu, X.-L. Flexible quasi-solid-state sodium-ion full battery with ultralong cycle life, high energy density and high-rate capability. Nano Res. 2022, 15, 925–932. [Google Scholar] [CrossRef]
- Wang, X.; Huang, H.; Zhou, F.; Das, P.; Wen, P.; Zheng, S.; Lu, P.; Yu, Y.; Wu, Z.-S. High-voltage aqueous planar symmetric sodium ion micro-batteries with superior performance at low-temperature of −40 ºC. Nano Energy 2021, 82, 105688. [Google Scholar] [CrossRef]








| Classification | Electrolyte | Cathode | Anode | Voltage Range(V) | Capacity (mAh/g) | Energy Density (Wh kg−1) | Ref. |
|---|---|---|---|---|---|---|---|
| Diluted Electrolyte | 0.5 M Na2SO4 | Na0.35MnO2 | ppy@MoO3 | 0–1.7 | 26 (0.55 A g−1) | 20 | [47] |
| 1 M Na2SO4 | Na2CuFe(CN)6 | NaTi2(PO4)3 | 0–1.8 | 103 (0.2 A g−1) | 48 | [48] | |
| Na0.44MnO2 | FePO4.2H2O | 0–1.2 | 70 (0.228 A g−1) | - | [49] | ||
| Na2VTi(PO4)3 | Na2VTi(PO4)3 | 0.2–1.5 | 58 (0.062 A g−1) | 30 | [29] | ||
| Na0.44MnO2 | NaV3(PO4)3 | 0.2–1.5 | 113 (1 C) | - | [50] | ||
| Na0.44MnO2 | NaTi2(PO4)3 | 0.1–1.3 | 103 (0.266 A g−1) | - | [51] | ||
| Na3V2(PO4)3 | NaTi2(PO4)3 | 0.5–1.6 | 71 (2 A g−1) | 36 | [52] | ||
| Na3MnTi(PO4)3 | Na3MnTi(PO4)3 | 0.4–1.8 | 57.9 (0.02935 A g−1) | 40 | [53] | ||
| Mn5O8 | Mn5O8 | 0.8–1.7 | 116 (5 A g−1) | 40 | [54] | ||
| Concentrated Electrolyte | 4 M NaClO4 | Na2FeP2O7 | NaTi2(PO4)3 | 0–1.4 | 43 (2 mA cm−2) | - | [55] |
| 5 M NaNO3 | NaVPO4F | polyimide | 0–1.8 | 40 (0.05 A g−1) | - | [31] | |
| 5 M NaClO4 | m-NiHCF | NaTi2(PO4)3@C | 0–1 | 61.4 (0.1 A g−1) | 86 | [56] | |
| WIS Electrolyte | 7 M NaTOF(H2O) + 8 M NATOF(PC) | Na3V2(PO4)3 | NaTi2(PO4)3 | 0.3–1.5 | 119 (0.12 A g−1) | 45 | [57] |
| 10 M NaClO4 + 2 vol.% VC | Na3V2O2x(PO4)2F3-2x/MWCNT | NaTi2(PO4)3-C | 1.0–1.8 | 40 (0.65 A g−1) | 65 | [25] | |
| Na4MnV(PO4)3-rGO | NaTi2(PO4)3-MWCNT | 0.8–1.65 | 97 (10 C–1.1 A g−1) | 130 | [58] | ||
| 17 M NaClO4 | Na2Zn3[Fe(CN)6]2 | Na3Fe3(PO4)4 | 0.4–1.8 | 80 (0.083 A g−1) | 46 | [59] | |
| Na2Mn[Fe(CN)6] | KMn[Cr(CN)6] | 0.8–2.6 | 34 | 58 | [60] | ||
| Na3V2(PO4)2F3-SWCNT | NaTi2(PO4)3-MWCNT | 0.6–2.1 | 75.2 (0.128 A g−1) | 150 | [61] | ||
| MnFe-PBA | CrCr-PBA | 0.5–2.5 | 52.8 (0.125 A g−1) | 81.6 | [62] | ||
| Na2FePO4F | NaTi2(PO4)3 | 0.2–1.8 | 90 (1 mA cm−2) | 30 | [52] | ||
| Na2MnFe(CN)6 | NaTi2(PO4)3 | 0.5–2 | 117 (2.0 mA cm−2) | - | [34] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, M.; Luo, J.; Guo, X.; Chen, J.; Cao, Y.; Chen, W. Aqueous Rechargeable Sodium-Ion Batteries: From Liquid to Hydrogel. Batteries 2022, 8, 180. https://doi.org/10.3390/batteries8100180
Yang M, Luo J, Guo X, Chen J, Cao Y, Chen W. Aqueous Rechargeable Sodium-Ion Batteries: From Liquid to Hydrogel. Batteries. 2022; 8(10):180. https://doi.org/10.3390/batteries8100180
Chicago/Turabian StyleYang, Mingrui, Jun Luo, Xiaoniu Guo, Jiacheng Chen, Yuliang Cao, and Weihua Chen. 2022. "Aqueous Rechargeable Sodium-Ion Batteries: From Liquid to Hydrogel" Batteries 8, no. 10: 180. https://doi.org/10.3390/batteries8100180