Photoacoustic Tomography with a Ring Ultrasound Transducer: A Comparison of Different Illumination Strategies
Abstract
:Featured Application
Abstract
1. Introduction
2. Material and Methods
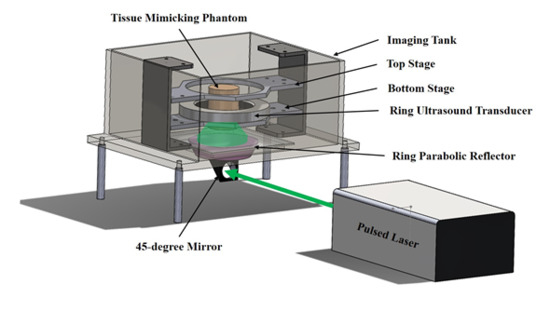
2.1. UST/PAT Acquisition System
2.2. Laser Source and Light Illumination Schemes
2.3. Tissue-Mimicking Phantoms
2.4. UST and PAT Image Reconstruction
3. Results and Discussion
3.1. A Comparison of the Three Different Illumination Methods
3.2. The PA Amplitude of the Targeted Cross-Section as a Function of Illumination Position
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA A Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [PubMed]
- Elmore, J.G.; Armstrong, K.; Lehman, C.D.; Fletcher, S.W. Screening for breast cancer. JAMA 2005, 293, 1245–1256. [Google Scholar] [CrossRef] [PubMed]
- Kuhl, C.K.; Schrading, S.; Leutner, C.C.; Wardelmann, E.; Fimmers, R.; Schild, H.H.; Morakkabati-Spitz, N.; Kuhn, W. Mammography, Breast Ultrasound, and Magnetic Resonance Imaging for Surveillance of Women at High Familial Risk for Breast Cancer. J. Clin. Oncol. 2005, 23, 8469–8476. [Google Scholar] [CrossRef] [PubMed]
- Tamimi, R.M.; Byrne, C.; Colditz, G.A.; Hankinson, S.E. Endogenous Hormone Levels, Mammographic Density, and Subsequent Risk of Breast Cancer in Postmenopausal Women. J. Natl. Cancer Inst. 2007, 99, 1178–1187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saslow, D.; Boetes, C.; Burke, W.; Harms, S.; Leach, M.O.; Lehman, C.D.; Morris, E.; Pisano, E.; Schnall, M.; Sener, S.; et al. American Cancer Society Guidelines for Breast Screening with MRI as an Adjunct to Mammography. Obstet. Gynecol. Surv. 2007, 62, 458–460. [Google Scholar] [CrossRef]
- Lee, C.H.; Dershaw, D.D.; Kopans, D.; Evans, P.; Monsees, B.; Monticciolo, D.; Brenner, R.J.; Bassett, L.; Berg, W.; Feig, S.; et al. Breast Cancer Screening With Imaging: Recommendations From the Society of Breast Imaging and the ACR on the Use of Mammography, Breast MRI, Breast Ultrasound, and Other Technologies for the Detection of Clinically Occult Breast Cancer. J. Am. Coll. Radiol. 2010, 7, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Jensen, J.A. Medical ultrasound imaging. Prog. Biophys. Mol. Biol. 2007, 93, 153–165. [Google Scholar] [CrossRef] [PubMed]
- Gordon, P.B.; Goldenberg, S.L. Malignant breast masses detected only by ultrasound. A retrospective review. Cancer 1995, 76, 626–630. [Google Scholar] [CrossRef]
- Madjar, H. Role of Breast Ultrasound for the Detection and Differentiation of Breast Lesions. Breast Care 2010, 5, 109–114. [Google Scholar] [CrossRef] [Green Version]
- Corsetti, V.; Houssami, N.; Ferrari, A.; Ghirardi, M.; Bellarosa, S.; Angelini, O.; Bani, C.; Sardo, P.; Remida, G.; Galligioni, E.; et al. Breast screening with ultrasound in women with mammography-negative dense breasts: Evidence on incremental cancer detection and false positives, and associated cost. Eur. J. Cancer 2008, 44, 539–544. [Google Scholar] [CrossRef]
- Kruger, R.A.; Lam, R.B.; Reinecke, D.R.; Del Rio, S.P.; Doyle, R.P. Photoacoustic angiography of the breast. Med. Phys. 2010, 37, 6096–6100. [Google Scholar] [CrossRef] [PubMed]
- Menke, J. Photoacoustic breast tomography prototypes with reported human applications. Eur. Radiol. 2015, 25, 2205–2213. [Google Scholar] [CrossRef] [PubMed]
- Mallidi, S.; Luke, G.P.; Emelianov, S. Photoacoustic imaging in cancer detection, diagnosis, and treatment guidance. Trends Biotechnol. 2011, 29, 213–221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mehrmohammadi, M.; Yoon, S.J.; Yeager, D.; Emelianov, S.Y. Photoacoustic Imaging for Cancer Detection and Staging. Curr. Mol. Imaging 2013, 2, 89–105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siphanto, R.I.; Thumma, K.K.; Kolkman, R.G.M.; Van Leeuwen, T.G.; De Mul, F.F.M.; Van Neck, J.W.; Van Adrichem, L.N.A.; Steenbergen, W. Serial noninvasive photoacoustic imaging of neovascularization in tumor angiogenesis. Opt. Express 2005, 13, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Brahimi-Horn, M.C.; Chiche, J.; Pouysségur, J. Hypoxia and cancer. J. Mol. Med. 2007, 85, 1301–1307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heijblom, M.; Klaase, J.M.; Engh, F.M.V.D.; Van Leeuwen, T.G.; Steenbergen, W.; Manohar, S. Imaging tumor vascularization for detection and diagnosis of breast cancer. Technol. Cancer Res. Treat. 2011, 10, 607–623. [Google Scholar] [CrossRef] [PubMed]
- Alcantara, D.; Leal, M.P.; García-Bocanegra, I.; García-Martín, M.L. Molecular imaging of breast cancer: Present and future directions. Front. Chem. 2014, 2, 112. [Google Scholar] [CrossRef] [PubMed]
- Luke, G.P.; Yeager, D.; Emelianov, S.Y. Biomedical applications of photoacoustic imaging with exogenous contrast agents. Ann. Biomed. Eng. 2012, 40, 422–437. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Jeevarathinam, A.S.; Humphries, K.; Jhunjhunwala, A.; Chen, F.; Hariri, A.; Miller, B.R.; Jokerst, J.V. A Mechanistic Investigation of Methylene Blue and Heparin Interactions and Their Photoacoustic Enhancement. Bioconjug. Chem. 2018, 29, 3768–3775. [Google Scholar] [CrossRef] [PubMed]
- Jeevarathinam, A.S.; Pai, N.; Huang, K.; Hariri, A.; Wang, J.; Bai, Y.; Wang, L.; Hancock, T.; Keys, S.; Penny, W.; et al. A cellulose-based photoacoustic sensor to measure heparin concentration and activity in human blood samples. Biosens. Bioelectron. 2019, 126, 831–837. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.V. Prospects of photoacoustic tomography. Med. Phys. 2008, 35, 5758–5767. [Google Scholar] [CrossRef] [PubMed]
- Ermilov, S.A.; Khamapirad, T.; Conjusteau, A.; Leonard, M.H.; Lacewell, R.; Mehta, K.; Miller, T.; Oraevsky, A.A. Laser optoacoustic imaging system for detection of breast cancer. J. Biomed. Opt. 2009, 14, 024007. [Google Scholar] [CrossRef] [PubMed]
- Kruger, R.A.; Kuzmiak, C.M.; Lam, R.B.; Reinecke, D.R.; Del Rio, S.P.; Steed, D. Dedicated 3D photoacoustic breast imaging. Med. Phys. 2013, 40, 113301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, L.; Hu, P.; Shi, J.; Appleton, C.M.; Maslov, K.; Li, L.; Zhang, R.; Wang, L.V. Single-breath-hold photoacoustic computed tomography of the breast. Nat. Commun. 2018, 9, 2352. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Hu, P.; Shi, J.; Maslov, K.I.; Appleton, C.M. Clinical photoacoustic computed tomography of the human breast in vivo within a single breath hold. In Photons Plus Ultrasound: Imaging and Sensing 2018; International Society for Optics and Photonics: Bellingham, WA, USA, 2018; Volume 10494, p. 104942X. [Google Scholar]
- Klosner, M.; Chan, G.; Wu, C.; Heller, D.F.; Su, R.; Ermilov, S.; Brecht, H.P.; Ivanov, V.; Talole, P.; Lou, Y.; et al. Advanced laser system for 3D optoacoustic tomography of the breast. In Photons Plus Ultrasound: Imaging and Sensing 2016; International Society for Optics and Photonics: Bellingham, WA, USA, 2016; Volume 9708, p. 97085B. [Google Scholar]
- Heijblom, M.; Piras, D.; Xia, W.; Van Hespen, J.C.G.; Engh, F.M.V.D.; Klaase, J.M.; Van Leeuwen, T.G.; Steenbergen, W.; Manohar, S. Imaging breast lesions using the Twente Photoacoustic Mammoscope: Ongoing clinical experience. In Photons Plus Ultrasound: Imaging and Sensing 2012; International Society for Optics and Photonics: Bellingham, WA, USA, 2012; Volume 8223, p. 82230C. [Google Scholar]
- Deng, Z.; Zhao, H.; Ren, Q.; Li, C. Acoustically penetrable optical reflector for photoacoustic tomography. J. Biomed. Opt. 2013, 18, 070503. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Li, C. Noninvasively measuring oxygen saturation of human finger-joint vessels by multi-transducer functional photoacoustic tomography. J. Biomed. Opt. 2016, 21, 61009. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Li, W. Slip-ring-based multi-transducer photoacoustic tomography system. Opt. Lett. 2016, 41, 2859–2862. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Lan, B.; Liu, W.; Xia, J.; Yao, J. Internal-illumination photoacoustic computed tomography. J. Biomed. Opt. 2018, 23, 1–4. [Google Scholar] [CrossRef]
- Bungart, B.; Cao, Y.; Yang-Tran, T.; Gorsky, S.; Lan, L.; Roblyer, D.; Koch, M.O.; Cheng, L.; Masterson, T.; Cheng, J.X. Cylindrical illumination with angular coupling for whole-prostate photoacoustic tomography. Biomed. Opt. Express 2019, 10, 1405–1419. [Google Scholar] [CrossRef]
- Duric, N.; Littrup, P.; Schmidt, S.; Li, C.P.; Roy, O.; Bey-Knight, L.; Janer, R.; Kunz, D.; Chen, X.Y.; Goll, J.; et al. Breast imaging with the SoftVue imaging system: First results. In Medical Imaging 2013: Ultrasonic Imaging, Tomography, and Therapy; SPIE Medical Imaging; International Society for Optics and Photonics: Bellingham, WA, USA, 2013. [Google Scholar]
- Duric, N.; Littrup, P.; Poulo, L.; Babkin, A.; Pevzner, R.; Holsapple, E.; Rama, O.; Glide, C. Detection of breast cancer with ultrasound tomography: First results with the Computed Ultrasound Risk Evaluation (CURE) prototype. Med. Phys. 2007, 34, 773–785. [Google Scholar] [CrossRef] [PubMed]
- Karpiouk, A.B.; Aglyamov, S.R.; Mallidi, S.; Shah, J.; Scott, W.G.; Rubin, J.M.; Emelianov, S.Y. Combined ultrasound and photoacoustic imaging to detect and stage deep vein thrombosis: Phantom and ex vivo studies. J. Biomed. Opt. 2008, 13, 054061. [Google Scholar] [CrossRef] [PubMed]
- Ranger, B.; Littrup, P.J.; Duric, N.; Chandiwala-Mody, P.; Li, C.P.; Schmidt, S.; Lupinacci, J. Breast ultrasound tomography versus MRI for clinical display of anatomy and tumor rendering: Preliminary results. Am. J. Roentgenol. 2012, 198, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Duric, N.; Littrup, P.; Babkin, A.; Chambers, D.; Azevedo, S.; Kalinin, A.; Pevzner, R.; Tokarev, M.; Holsapple, E.; Rama, O.; et al. Development of ultrasound tomography for breast imaging: Technical assessment. Med. Phys. 2005, 32, 1375–1386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alshahrani, S.; Yan, Y.; Avrutsky, I.; Anastasio, M.; Malyarenko, E.; Duric, N.; Mehrmohammadi, M. Design and development of a full-ring ultrasound and photoacoustic tomography system for breast cancer imaging. In Proceedings of the 2017 IEEE International Ultrasonics Symposium (IUS), Washington, DC, USA, 6–9 September 2017. [Google Scholar]
- Alshahrani, S.; Pattyn, A.; Alijabbari, N.; Yan, Y.; Anastasio, M.; Mehrmohammadi, M. The Effectiveness of the Omnidirectional Illumination in Full-Ring Photoacoustic Tomography. In Proceedings of the 2018 IEEE International Ultrasonics Symposium (IUS), Kobe, Japan, 22–25 October 2018. [Google Scholar]
- Alshahrani, S.S.; Yan, Y.; Malyarenko, E.; Avrutsky, I.; Anastasio, M.A.; Mehrmohammadi, M. An advanced photoacoustic tomography system based on a ring geometry design. In Medical Imaging 2018: Ultrasonic Imaging and Tomography; International Society for Optics and Photonics: Bellingham, WA, USA, 2018; Volume 10580, p. 1058005. [Google Scholar]
- Alshahrani, S.S.; Yan, Y.; Alijabbari, N.; Pattyn, A.; Avrutsky, I.; Malyarenko, E.; Poudel, J.; Anastasio, M.; Mehrmohammadi, M. All-reflective ring illumination system for photoacoustic tomography. J. Biomed. Opt. 2019, 24, 046004. [Google Scholar]
- Maggi, L.; Cortela, G.; von Kruger, M.A.; Negreira, C.; de Albuquerque Pereira, W.C. Ultrasonic Attenuation and Speed in phantoms made of PVCP and Evaluation of acoustic and thermal properties of ultrasonic phantoms made of polyvinyl chloride-plastisol (PVCP). In Proceedings of the IWBBIO, Granada Spain, 12–20 March 2013. [Google Scholar]
- Li, C.; Sandhu, G.Y.; Boone, M.; Duric, N. Breast imaging using waveform attenuation tomography. In Medical Imaging 2017: Ultrasonic Imaging and Tomography; International Society for Optics and Photonics: Bellingham, WA, USA, 2017; Volume 10139, p. 101390A. [Google Scholar]
- Xu, M.; Wang, L.V. Universal back-projection algorithm for photoacoustic computed tomography. Phys. Rev. E 2005, 71, 016706. [Google Scholar] [CrossRef] [Green Version]







© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alijabbari, N.; Alshahrani, S.S.; Pattyn, A.; Mehrmohammadi, M. Photoacoustic Tomography with a Ring Ultrasound Transducer: A Comparison of Different Illumination Strategies. Appl. Sci. 2019, 9, 3094. https://doi.org/10.3390/app9153094
Alijabbari N, Alshahrani SS, Pattyn A, Mehrmohammadi M. Photoacoustic Tomography with a Ring Ultrasound Transducer: A Comparison of Different Illumination Strategies. Applied Sciences. 2019; 9(15):3094. https://doi.org/10.3390/app9153094
Chicago/Turabian StyleAlijabbari, Naser, Suhail S. Alshahrani, Alexander Pattyn, and Mohammad Mehrmohammadi. 2019. "Photoacoustic Tomography with a Ring Ultrasound Transducer: A Comparison of Different Illumination Strategies" Applied Sciences 9, no. 15: 3094. https://doi.org/10.3390/app9153094