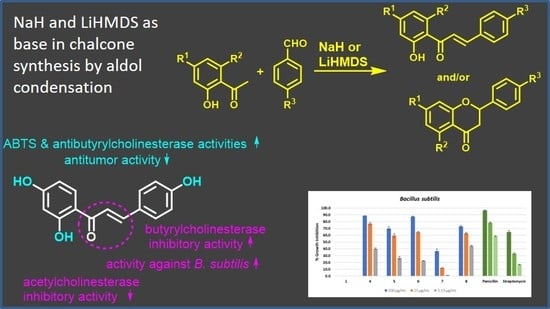
Chalcones and Flavanones Bearing Hydroxyl and/or Methoxyl Groups: Synthesis and Biological Assessments
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Methods
2.2. Synthesis of Chalcones and Flavanones
- (a)
- The benzylation of the hydroxyl groups: the 4′ and 6′-hydroxyl groups in the starting material 2′,4′,6′-trihydroxyacetophenone were protected using the methodology described by Figueiredo [32]. Briefly, the 2′,4′,6′-trihydroxyacetophenone (2.7 g, 16.1 mmol), dissolved in a minimum amount of dry dimethylformamide (DMF) (~20 mL), was mixed with K2CO3 (6 equivalents) under constant stirring. Then, benzyl bromide (3 equivalents) was added and the reaction was performed at 150 °C under reflux for 2 h. After that, the reaction mixture was filtered to remove the K2CO3 and the inorganic salts washed with DMF. The filtrate was poured over crushed ice and the mixture acidified to pH < 5 with HCl 20%. The precipitated 4′,6′-dibenzyloxy-2′-hydroxyacetophenone was filtered and crystallized from ethanol (4.75 g, 85% yield).
- (b)
- The aldol condensation: The synthesis of 4′,6′-dibenzyloxy-2′-hydroxy-4-methoxychalcone 2 was performed by dissolving 4′,6′-benzyloxy-2′-hydroxyacetophenone (1.5566 g) in dried THF and was then mixed with NaH (2.5 equivalents). After 10 min of stirring under nitrogen atmosphere at room temperature, 4-methoxybenzaldehyde (1.2 equivalents) was added. The reaction was finished after 3 h by pouring over crushed ice and addition of HCl 37% to pH < 2. The precipitate was filtered and washed with water, and the crude product was crystallized from acetone to afford 4′,6′-dibenzyloxy-2′-hydroxy-4-methoxychalcone 2 (1.6573 g, 80% yield).
- (c)
- The benzyl group’s cleavage: The final step was deprotecting the hydroxyl groups at 4′ and 6′ positions by cleavage of the benzyl groups. This procedure was adapted from the method described by Gomes et al. [33]. Briefly, 4′,6′-dibenzyloxy-2′-hydroxy-4-methoxychalcone 2 (567.0 mg) was mixed with 40 mL of a mixture of HCl/Acetic acid (1:10) under stirring at 80 °C during 13 h. The reaction was finished by pouring the mixture over crushed ice, the solid formed was washed with water until pH ~ 5 and then purified by TLC, eluting it twice in CH2Cl2, affording 5,7-dihydroxy-4′-methoxy-flavanone 4 (138.7 mg, 59%).
- (a)
- The benzylation of the hydroxyl groups: the 4-hydroxybenzaldehyde (1.3 g, 10.6 mmol) was dissolved in a minimum of dry dimethylformamide (DMF) (~15 mL), and it was mixed with K2CO3 (3 equivalents) under constant stirring. Then, benzyl bromide (1.5 equivalents) was added, and the reaction was performed at 150 °C under reflux for 2 h. After that, the reaction mixture was filtered to remove the K2CO3 and washed with DMF. The filtrate was poured over crushed ice and HCl 20% added until pH < 5. The precipitated 4-benzyloxybenzaldehyde was filtered and crystallized from ethanol (1.8 g, 78%).
- (b)
- The aldol condensation: The 2′-hydroxy-4′,6′-dimethoxyacetophenone (898.3 mg) was dissolved in dried THF (~15 mL) and mixed with NaH (2.5 equivalents) at room temperature and under N2 atmosphere. After 10 min, 4-benzyloxybenzaldehyde (1.2 equivalents) was added, and the reaction was finished after 4 h by precipitation over crushed ice acidified with HCl 37% to pH < 2. 4-Benzyloxy-2′-hydroxy-4′,6′-dimethoxychalcone 3 was obtained by crystallization from ethanol (1.433 g, 80%).
- (c)
- The benzyl group’s cleavage: The chalcone 3 (330.6 mg) was mixed with 30 mL of a mixture of HCl/Acetic acid (1:10) under stirring at 55 °C during 60 h. The reaction was finished by pouring the mixture over crushed ice, the formed solid was washed with water until pH ~5 and purified by TLC using hexane/ethyl acetate (1:1) as eluent, affording 4′-hydroxy-5,7-dimethoxyflavanone 5 as pale-yellow amorphous powder (21.0 mg, 8%).
2.3. Biological Activities
2.3.1. DPPH Scavenging Activity
2.3.2. ABTS Scavenging Activity
2.3.3. Anticholinesterasic Activity
2.3.4. Antimicrobial Activity
2.3.5. Antitumor Activity
3. Results and Discussion
3.1. Chalcones and Flavanones Synthesis
3.2. Biological Evaluations
3.2.1. Antioxidant Activity
3.2.2. Anticholinesterasic Activity
3.2.3. Antimicrobial Activity
3.2.4. Antitumor Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Miranda, C.L.; Maier, C.S.; Stevens, J.F. Flavonoids; John Wiley & Sons Ltd.: Chichester, UK, 2012. [Google Scholar] [CrossRef]
- Santos, E.L.; Maia, B.H.S.; Ferriani, A.P.; Teixeira, S.D. Flavonoids: Classification, biosynthesis and chemical ecology. In Flavonoids–from Biosynthesis to Human Health; Justino, G.C., Ed.; Intech: London, UK, 2017; pp. 3–16. ISBN 978-953-51-3424-4. [Google Scholar]
- Khan, M.K.; Huma, Z.E.; Dangles, O. A comprehensive review on flavanones, the major citrus polyphenols. J. Food Compos. Anal. 2014, 33, 85–104. [Google Scholar] [CrossRef]
- Matos, M.J.; Vasquez-Rodriguez, S.; Uriarte, E.; Santana, L. Potential pharmacological uses of chalcones: A patent review (from June 2011-2014). Expert Opin. Ther. Patents 2014, 25, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Aoki, N.; Muko, M.; Ohta, E.; Ohta, S. C-Geranylated chalcones from the stems of Angelica keiskei with superoxide-scavenging activity. J. Nat. Prod. 2008, 71, 1308–1310. [Google Scholar] [CrossRef] [PubMed]
- Mahapatra, D.K.; Bharti, S.K.; Asati, V. Anti-cancer chalcones: Structural and molecular target perspectives. Eur. J. Med. Chem. 2015, 98, 69–114. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Chen, G.; Lu, X.; Wang, S.; Hans, S.; Li, Y.; Ping, G.; Jiang, X.; Li, H.; Yang, J.; et al. Novel chalcone derivatives as hypoxia-inducible factor (HIF)-1 inhibitor: Synthesis, anti-invasive and anti-angiogenic properties. Eur. J. Med. Chem. 2015, 89, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Winter, E.; Locatelli, C.; Di Pietro, A.; Creczynski-Pasa, T.B. Recent trends of chalcones potentialities as antiproliferative and antiresistance agents. Anticancer Agents Med. Chem. 2015, 15, 592–604. [Google Scholar] [CrossRef] [PubMed]
- Mascarello, A.; Chiaradia, L.D.; Vernal, J.; Villarino, A.; Guido, R.V.; Perizzolo, P.; Poirier, V.; Wong, D.; Martins, P.G.; Nunes, R.J.; et al. Inhibition of Mycobacterium tuberculosis tyrosine phosphatase PtpA by synthetic chalcones: Kinetics, molecular modeling, toxicity and effect on growth. Bioorg. Med. Chem. 2010, 18, 3783–3789. [Google Scholar] [CrossRef] [PubMed]
- Tomar, V.; Bhattacharjee, G.; Kamaluddin, K.; Rajakumar, S.; Srivastava, K.; Puri, S.K. Synthesis of new chalcone derivatives containing acridinyl moiety with potential antimalarial activity. Eur. J. Med. Chem. 2010, 45, 745–751. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, S.U.F.; Siddiqui, H.L.; Johns, M.; Detorio, M.; Schinazi, R.F. Anti-HIV-1 and cytotoxicity studies of piperidyl-thienyl chalcones and their 2-pyrazoline derivatives. Med. Chem. Res. 2012, 21, 3741–3749. [Google Scholar] [CrossRef]
- Abdullah, M.I.; Mahmood, A.; Madni, M.; Masood, S.; Kashif, M. Synthesis, characterization, theoretical, anti-bacterial and molecular docking studies of quinoline based chalcones as a DNA gyrase inhibitor. Bioorg. Chem. 2014, 54, 31–37. [Google Scholar] [CrossRef]
- Birari, R.B.; Gupta, S.; Mohan, C.G.; Bhutani, K.K. Antiobesity and lipid lowering effects of glycyrrhizachalcones: Experimental and computational studies. Phytomedicine 2011, 18, 795–801. [Google Scholar] [CrossRef] [PubMed]
- Kantevari, S.; Addla, D.; Bagul, P.K.; Sridhar, B.; Banerjee, S.K. Synthesis and evaluation of novel 2-butyl-4-chloro-1-methylimidazole embedded chalcones and pyrazoles as angiotensin converting enzyme (ACE) inhibitors. Bioorg. Med. Chem. 2011, 19, 4772–4781. [Google Scholar] [CrossRef] [PubMed]
- Sashidhara, K.V.; Palnati, G.R.; Sonkar, R.; Avula, S.R.; Awasthi, C.; Bhatia, G. Coumarinchalcone fibrates: A new structural class of lipid lowering agents. Eur. J. Med. Chem. 2013, 64, 422–431. [Google Scholar] [CrossRef] [PubMed]
- Mahapatra, D.K.; Asati, V.; Bharti, S.K. Chalcones and their role in management of diabetes mellitus: Structural and pharmacological perspectives. Eur. J. Med. Chem. 2015, 92, 839–865. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Anand, A.; Kumar, V. Recent developments in biological activities of chalcones: A mini review. Eur. J. Med. Chem. 2014, 85, 758–777. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Available online: http://www.who.int/news-room/fact-sheets/detail/cancer (accessed on 19 January 2019).
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics. CA Cancer J. Clin. 2017, 67, 7–30. [Google Scholar] [CrossRef]
- Cassini, A.; Högberg, L.D.; Plachouras, D.; Quattrocchi, A.; Hoxha, A.; Simonsen, G.S.; Colomb-Cotinat, M.; Kretzschmar, M.E.; Devleesschauwer, B.; Cecchini, M.; et al. Attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in the EU and the European Economic Area in 2015: A population-level modelling analysis. Lancet Infect. Dis. 2019, 19, 56–66. [Google Scholar] [CrossRef]
- Tillotson, G.S.; Zinner, S.H. Burden of antimicrobial resistance in an era of decreasing susceptibility. Expert Rev. Anti Infect. Ther. 2017, 15, 663–676. [Google Scholar] [CrossRef]
- Maresova, P.; Mohelska, H.; Dolejs, J.; Kuca, K. Socio-economic aspects of Alzheimer’s disease. Curr. Alzheimer Res. 2015, 12, 903–911. [Google Scholar] [CrossRef]
- Findley, L.J. The economic impact of Parkinson’s disease. Parkinsonism Relat. Disord. 2007, 13, 8–12. [Google Scholar] [CrossRef]
- Saeidnia, S.; Abdollahi, M. Toxicological and pharmacological concerns on oxidative stress and related diseases. Toxicol. Appl. Pharmacol. 2013, 273, 442–455. [Google Scholar] [CrossRef] [PubMed]
- Bukhari, S.N.A.; Jasamai, M.; Jantan, I.; Ahmad, W. Review of methods and various catalysts used for chalcone synthesis. Mini Rev. Org. Chem. 2013, 10, 73–83. [Google Scholar] [CrossRef]
- Ugwu, D.I.; Ezema, B.E.; Okoro, U.C.; Eze, F.U.; Ekoh, O.C.; Egbujor, M.C.; Ugwuja, D.I. Synthesis and pharmacological applications of chalcones: A review. Int. J. Chem. Sci. 2015, 13, 459–500. [Google Scholar]
- Hsieh, C.T.; Hsieh, T.J.; El-Shazly, M.; Chuang, D.W.; Tsai, Y.H.; Yen, C.T.; Wu, S.F.; Wu, Y.C.; Chang, F.R. Synthesis of chalcone derivatives as potential anti-diabetic agents. Bioorg. Med. Chem. Lett. 2012, 22, 3912–3915. [Google Scholar] [CrossRef] [PubMed]
- Perrin, C.L.; Chang, K.L. The complete mechanism of an aldol condensation. J. Org. Chem. 2016, 81, 5631–5635. [Google Scholar] [CrossRef] [PubMed]
- Clayden, J.; Greeves, N.; Warren, S. Organic Chemistry, 2nd ed.; Oxford University Press: Oxford, UK; New York, NY, USA, 2012; pp. 141–162. ISBN 978-0199270293. [Google Scholar]
- Mellado, M.; Madrid, A.; Martinéz, U.; Mella, J.; Salas, C.O.; Cuellar, M. Hansch’s analysis application to chalcone synthesis by Claisen-Schmidt reaction based DFT methodology. Chem. Pap. 2018, 72, 703–709. [Google Scholar] [CrossRef]
- Dharmaratne, H.R.W.; Nanayakkara, N.P.D.; Khan, I.A. Kavalactones from Piper methysticum, and their 13C NMR spectroscopic analyses. Phytochemistry 2002, 59, 429–433. [Google Scholar] [CrossRef]
- Figueiredo, A.G.P.R. Transformações de Cromona-3-Carbaldeído e de (E)-o-Dihidroxi-2-Estirilcromonas. Ph.D. Thesis, University of Aveiro, Aveiro, Portugal, July 2008. [Google Scholar]
- Gomes, A.; Neuwirth, O.; Freitas, M.; Couto, D.; Ribeiro, D.; Figueiredo, A.G.P.R.; Silva, A.M.S.; Seixas, R.S.G.R.; Pinto, D.C.G.A.; Tomé, A.C.; et al. Synthesis and antioxidant properties of new chromone derivatives. Bioorg. Med. Chem. 2009, 17, 7218–7226. [Google Scholar] [CrossRef]
- Cavaleiro, J.A.S. Isosakuranetin, a flavonone from Artemisia compestris subsp. maritima. Fitoterapia 1986, 57, 278–279. [Google Scholar]
- Silva, A.M.S.; Tavares, H.R.; Barros, A.I.N.R.A.; Cavaleiro, J.A.S. NMR and structural and conformational features of 2′-hydroxychalcones and flavones. Spectrosc. Lett. 1997, 30, 1655–1667. [Google Scholar] [CrossRef]
- Silva, A.M.S.; Cavaleiro, J.A.S.; Tarrago, G.; Marzin, C. Synthesis and characterization of ruthenium(II) complexes of 2’-hydroxychalcones. New J. Chem. 1999, 23, 329–335. [Google Scholar] [CrossRef]
- Blois, M. Antioxidant determinations by the use of a stable free radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Ellman, G.; Courtney, K.; Andres, V.; Featherstone, R. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Arruda, M.; Viana, H.; Rainha, N.; Neng, N.; Rosa, J.; Nogueira, J.; Barreto, M.C. Anti-acetylcholinesterase and antioxidant activity of essential oils from Hedychium gardnerianum Sheppard ex Ker-Gawl. Molecules 2012, 17, 3082–3092. [Google Scholar] [CrossRef] [PubMed]
- De León, L.; Beltrán, B.; Moujir, L. Antimicrobial activity of 6-oxophenolic triterpenoids. Mode of action against Bacillus subtilis. Planta Med. 2005, 71, 313–319. [Google Scholar] [CrossRef]
- Moujir, L.L.; Gouveia, V.; Toubarro, D.; Barreto, M.C. Determination of Cytotoxicity Against Tumour Cell Lines. In Determination of Biological Activities; Barreto, M.C., Simões, N., Eds.; Azores University: Ponta Delgada, Portugal, 2012; pp. 41–62. [Google Scholar]
- Rocha, D.H.A.; Pinto, D.C.G.A.; Silva, A.M.S. One-pot synthesis of 3-methylflavones and their transformation into (E)-3-styrilflavones via Wittig reactions. Synlett 2013, 24, 2683–2686. [Google Scholar] [CrossRef]
- Gan, L.S.; Zeng, L.W.; Li, X.R.; Zhou, C.X.; Li, J. New homoisoflavonoid analogues protect cells by regulating autophagy. Bioorg. Med. Chem. Lett. 2017, 27, 1441–1445. [Google Scholar] [CrossRef]
- Aköz, B.E.; Ertan, R. Chemical and structural properties of chalcones I. FABAD J. Pharm. Sci. 2011, 36, 223–242. [Google Scholar]
- Cushman, M.; Nagarathnan, D. A method for the facile synthesis of ring-A hydroxylated flavones. Tetrahedron Lett. 1990, 31, 6497–6500. [Google Scholar] [CrossRef]
- Vaz, P.A.A.M.; Pinto, D.C.G.A.; Rocha, D.H.A.; Silva, A.M.S.; Cavaleiro, J.A.S. New synthesis of 3-aroylflavone derivatives; Knoevenagel condensation and oxidation versus one-pot synthesis. Synlett 2012, 23, 2353–2356. [Google Scholar] [CrossRef]
- Aida, K.; Tawata, M.; Shindo, H.; Onaya, T.; Sasaki, H.; Yamaguchi, T.; Chin, M.; Mitsuhashi, H. Isoliquiritigenin: A new aldose reductase inhibitor from glycyrrhizae radix. Planta Med. 1990, 56, 254–258. [Google Scholar] [CrossRef] [PubMed]
- Suksamrarn, A.; Chotipong, A.; Suavansri, T.; Boongird, S.; Timsuksai, P.; Vimuttipong, S.; Chuaynugul, A. Antimycobacterial activity and cytotoxicity of flavonoids from the flowers of Chromolaena odorata. Arch. Pharm. Res. 2004, 27, 507–511. [Google Scholar] [CrossRef] [PubMed]
- Shoja, M. Crystal structure of 4’-hydroxy-5,7-dimethoxyflavanone, C17H16O5. Z. Kristallogr. New Cryst. Struct. 1997, 212, 127–128. [Google Scholar] [CrossRef]
- Engels, N.S.; Waltenberger, B.; Michalak, B.; Huynh, L.; Tran, H.; Kiss, A.K.; Stuppner, H. Inhibition of pro-inflammatory functions of human neutrophils by constituents of Melodorum fruticosum leaves. Chem. Biodivers. 2018, 15, e1800269. [Google Scholar] [CrossRef] [PubMed]
- Khamsan, S.; Liawruangrath, S.; Teerawutkulrag, A.; Pyne, S.G.; Garson, M.J.; Liawruangrath, B. The isolation of bioactive flavonoids from Jacaranda obtusifolia H.B.K. ssp. rhombifolia (G.F.W. Meijer) Gentry. Acta Pharm. 2012, 62, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Badarinath, A.V.; Rao, K.M.; Chetty, C.M.S.; Ramkanth, S.; Rajan, T.V.S.; Gnanaprakash, K. A review of in vitro antioxidant methods: Comparisons, correlations and considerations. Int. J. PharmTech Res. 2010, 2, 1276–1285. [Google Scholar]
- Nimse, S.B.; Pal, D. Free radicals, natural antioxidants and their reaction mechanisms. RSC Adv. 2015, 5, 27986–28006. [Google Scholar] [CrossRef]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Antioxidant properties of phenolic compounds. Trends Plant Sci. 1997, 2, 152–159. [Google Scholar] [CrossRef]
- Rossi, M.; Caruso, F.; Crespi, E.J.; Pedersen, J.Z.; Nakano, G.; Duong, M.; Mckee, C.; Lee, S.; Jiwrajka, M.; Caldwell, C.; et al. Probing antioxidant activity of 2′-hydroxychalcones: Crystal and molecular structures, in vitro antiproliferative studies and in vivo effects on glucose regulation. Biochimie 2013, 95, 1954–1963. [Google Scholar] [CrossRef]
- Cerretani, L.; Bendini, A. Rapid assays to evaluate the antioxidant capacity of phenols in virgin olive oil. In Olives and Olive Oil in Health and Disease Prevention; Preedy, V.R., Watson, R.R., Eds.; Elsevier: Albany, NY, USA, 2010; pp. 625–635. ISBN 9780123744203. [Google Scholar]
- Chokchaisiri, R.; Suaisom, C.; Sriphota, S.; Chindaduang, A.; Chuprajob, T.; Suksamrarn, A. Bioactive flavonoids of the flowers of Butea monosperma. Chem. Pharm. Bull. 2009, 57, 428–432. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.L.; Kuo, P.O.; Chiang, L.C.; Lin, C.C. Isoliquiritigenin inhibits the proliferation and induces apoptosis of human non-small lung cancer A549 cells. Clin. Exp. Pharmacol. Physiol. 2004, 31, 414–418. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xie, S.; Liu, C.; Wu, Y.; Liu, Y.; Cai, Y. Inhibitory effect of liquiritigenin on migration via downregulation ProMMP-2 and PI3K/Akt signaling pathway in human lung adenocarcinoma A549 cells. Nutr. Cancer 2012, 64, 627–634. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.H.; Li, T.; Tong, Y.G.; Chen, X.J.; Chen, X.P.; Wang, Y.W.; Lu, J.J. A systematic review of the anticancer properties of compound isolated from licorice (Gancao). Planta Med. 2015, 81, 1670–1687. [Google Scholar] [CrossRef] [PubMed]
- Lieberman, M.M.; Patterson, G.M.L.; Moore, R.E. In vitro bioassays for anticancer drug screening: Effects of cell concentration and other assay parameters on growth inhibitory activity. Cancer Lett. 2001, 173, 21–29. [Google Scholar] [CrossRef]
- Marinho, J.; Pedro, M.; Pinto, D.C.G.A.; Silva, A.M.S.; Cavaleiro, J.A.S.; Sunkel, C.E.; Nascimento, M.S.J. 4′-Methoxy-2-styrylchromone a novel microtubule-stabilizing antimitotic agente. Biochem. Pharmacol. 2008, 75, 826–835. [Google Scholar] [CrossRef]









| Compounds and References | IC50 μg/mL (± SD) | |
|---|---|---|
| DPPH | ABTS | |
| 1 | >150 | >150 |
| 4 | >150 | 140.15 (±0.03) a |
| 5 | >150 | 85.47 (±0.15) b |
| 6 | 26.47 (±0.70) a | 12.72 (±0.92) c |
| 7 | >150 | >150 |
| 8 | >150 | >150 |
| Trolox | 7.25 (±0.09) b | 2.68 (±0.08) d |
| Quercetin | 3.01 (±0.03) c | 0.57 (±0.02) e |
| Compounds and References | IC50 μg/mL (± SD) | ||
|---|---|---|---|
| M. luteus | B. subtilis | E. coli | |
| 1 | >200 | >200 | >200 |
| 4 | 164.89 (±2.97) a | 76.46 (±4.27) a | >200 |
| 5 | >200 | >200 | >200 |
| 6 | 23.78 (±2.04) b | 9.33 (±0.25) b | >200 |
| 7 | 23.64 (±2.24) b | 20.48 (±3.13) c | >200 |
| 8 | >200 | 74.64 (±3.92) a | >200 |
| Penicillin | 8.47 (±0.55) c | 0.28 (±0.02) d | >40 |
| Streptomycin | 0.59 (±0.02) d | 15.86 (±0.21) e | 14.45 (±1.73) |
| Compounds and References | IC50 μg/mL (± SD) |
|---|---|
| A549 Cell Line | |
| 1 | 101.23 (±0.42) a |
| 4 | 135.89 (±2.61) b |
| 5 | 93.42 (±2.42) c |
| 6 | >200 |
| 7 | >200 |
| 8 | 93.31 (±2.45) c |
| Paclitaxel | 5.96 (±0.48) d |
| Colchicine | 2.78 (±0.71) d |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosa, G.P.; Seca, A.M.L.; Barreto, M.d.C.; Silva, A.M.S.; Pinto, D.C.G.A. Chalcones and Flavanones Bearing Hydroxyl and/or Methoxyl Groups: Synthesis and Biological Assessments. Appl. Sci. 2019, 9, 2846. https://doi.org/10.3390/app9142846
Rosa GP, Seca AML, Barreto MdC, Silva AMS, Pinto DCGA. Chalcones and Flavanones Bearing Hydroxyl and/or Methoxyl Groups: Synthesis and Biological Assessments. Applied Sciences. 2019; 9(14):2846. https://doi.org/10.3390/app9142846
Chicago/Turabian StyleRosa, Gonçalo P., Ana M. L. Seca, Maria do Carmo Barreto, Artur M. S. Silva, and Diana C. G. A. Pinto. 2019. "Chalcones and Flavanones Bearing Hydroxyl and/or Methoxyl Groups: Synthesis and Biological Assessments" Applied Sciences 9, no. 14: 2846. https://doi.org/10.3390/app9142846