Perovskite Thin Film Materials Stabilized and Enhanced by Zinc(II) Doping
Abstract
:Featured Application
Abstract
1. Introduction: Perovskite Solar Cells on the Rise
2. Doping of Perovskites with Mono- and Tri-Valent Metals
3. Doping of Perovskites with Zinc(II)
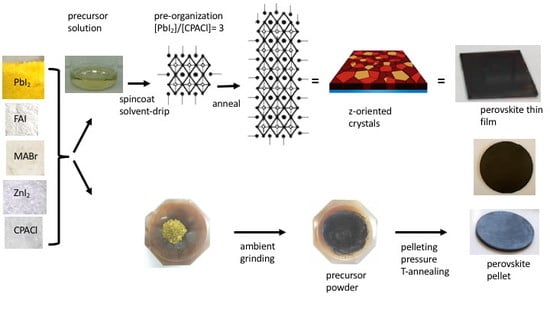
4. Influence of Zinc(II) on the Crystallization Mechanism
5. Optical Properties, Band Bending and Device Improvement
6. Discussion
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Polman, A.; Knight, M.; Garnett, E.C.; Ehrler, B.; Sinke, W.C. Photovoltaic materials: Present efficiencies and future challenges. Science 2016, 352, aad4424. [Google Scholar] [CrossRef]
- NREL Efficiency Chart. This Plot Is Courtesy of the National Renewable Energy Laboratory, Golden, CO. Available online: https://www.nrel.gov/pv/assets/pdfs/best-reserch-cell-efficiencies.20190411.pdf (accessed on 14 April 2019).
- Correa-Baena, J.P.; Abate, A.; Saliba, M.; Tress, W.; Jesper Jacobsson, T.; Grätzel, M.; Hagfeldt, A. The rapid evolution of highly efficient perovskite solar cells. Energy Environ. Sci. 2017, 10, 710–727. [Google Scholar] [CrossRef]
- Prakash, J.; Singh, A.; Sathiyan, G.; Ranjan, R.; Singh, A.; Garg, A.; Gupta, R.K. Progress in tailoring perovskite based solar cells through compositional engineering: Materials properties, photovoltaic performance and critical issues. Mater. Today Energy 2018, 9, 440–486. [Google Scholar] [CrossRef]
- Jeon, N.J.; Noh, J.H.; Yang, W.S.; Kim, Y.C.; Ryu, S.; Seo, J.; Seok, S. Il Compositional engineering of perovskite materials for high-performance solar cells. Nature 2015, 517, 476–480. [Google Scholar] [CrossRef]
- Weber, D. CH3NH3PbX3, a Pb(II)-System with cubic perovskite structure. Z. Naturforsch. B J. Chem. Sci. 1978, 1445, 1443–1445. [Google Scholar] [CrossRef]
- Abdelhady, A.L.; Saidaminov, M.I.; Murali, B.; Adinolfi, V.; Voznyy, O.; Katsiev, K.; Alarousu, E.; Comin, R.; Dursun, I.; Sinatra, L.; et al. Heterovalent dopant incorporation for bandgap and type engineering of perovskite crystals. J. Phys. Chem. Lett. 2016, 7, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Hao, F.; Stoumpos, C.C.; Cao, D.H.; Chang, R.P.H.; Kanatzidis, M.G. Lead-free solid-state organic-inorganic halide perovskite solar cells. Nat. Photonics 2014, 8, 489–494. [Google Scholar] [CrossRef]
- Klug, M.T.; Osherov, A.; Haghighirad, A.A.; Stranks, S.D.; Brown, P.R.; Bai, S.; Wang, J.T.W.; Dang, X.; Bulović, V.; Snaith, H.J.; et al. Tailoring metal halide perovskites through metal substitution: Influence on photovoltaic and material properties. Energy Environ. Sci. 2017, 10, 236–246. [Google Scholar] [CrossRef]
- Van Aert, S.; Geuchies, J.J.; van den Bos, K.H.W.; de Mello Donega, C.; van der Stam, W.; Meeldijk, J.D.; Bals, S.; Altantzis, T.; Vanmaekelbergh, D. Highly emissive divalent-ion-doped colloidal CsPb1–xM xBr3 perovskite nanocrystals through cation exchange. J. Am. Chem. Soc. 2017, 139, 4087–4097. [Google Scholar] [CrossRef]
- Jin, J.; Li, H.; Chen, C.; Zhang, B.; Xu, L.; Dong, B.; Song, H.; Dai, Q. Enhanced performance of perovskite solar cells with zinc chloride additives. ACS Appl. Mater. Interfaces 2017, 9, 42875–42882. [Google Scholar] [CrossRef]
- Green, M.A.; Ho-Baillie, A.; Snaith, H.J. The emergence of perovskite solar cells. Nat. Photonics 2014, 8, 506–514. [Google Scholar] [CrossRef]
- Tian, H.; Zhu, Y.; Hou, D.; Hu, Z.; Zeng, Z.; Lu, C.; Han, L.; Zhang, J.; Liu, P.; Chen, R.; et al. Zinc ion as effective film morphology controller in perovskite solar cells. Sustain. Energy Fuels 2018, 1093–1100. [Google Scholar] [CrossRef]
- Zheng, H.; Liu, G.; Xu, X.; Alsaedi, A.; Hayat, T.; Pan, X.; Dai, S. Acquiring high-performance and stable mixed-dimensional perovskite solar cells by using a transition-metal-substituted pb precursor. ChemSusChem 2018, 11, 3269–3275. [Google Scholar] [CrossRef]
- Zhao, W.; Yang, D.; Yang, Z.; Liu, S.F. Zn-doping for reduced hysteresis and improved performance of methylammonium lead iodide perovskite hybrid solar cells. Mater. Today Energy 2017, 5, 205–213. [Google Scholar] [CrossRef]
- Muscarella, L.A.; Petrova, D.; Cervasio, R.J.; Farawar, A.; Lugier, O.; McLure, C.; Slaman, M.J.; Wang, J.; von Hauff, E.; Williams, R.M. Enhanced grain-boundary emission lifetime and additive induced crystal orientation in one-step spin-coated mixed cationic (FA/MA) lead perovskite thin films stabilized by zinc iodide doping. Chem. Rxiv. Preprint 2017. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, J.; Bakr, O.M.; Sun, H.T. Metal-doped lead halide perovskites: Synthesis, properties, and optoelectronic applications. Chem. Mater. 2018, 30, 6589–6613. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhou, Z.; Chen, M.; Zong, Y.; Huang, J.; Pang, S.; Padture, N.P. Doping and alloying for improved perovskite solar cells. J. Mater. Chem. A 2016, 4, 17623–17635. [Google Scholar] [CrossRef]
- Li, B.; Li, Y.; Zheng, C.; Gao, D.; Huang, W. Advancements in the stability of perovskite solar cells: Degradation mechanisms and improvement approaches. RSC Adv. 2016, 6, 38079–38091. [Google Scholar] [CrossRef]
- Arora, N.; Grätzel, M.; Hu, Y.; Sadhanala, A.; Franckevičius, M.; Nazeeruddin, M.K.; Zakeeruddin, S.M.; Dar, M.I.; Friend, R.H.; Abdi-Jalebi, M.; et al. Impact of monovalent cation halide additives on the structural and optoelectronic properties of CH3NH3PbI3 Perovskite. Adv. Energy Mater. 2016, 6, 1502472. [Google Scholar] [CrossRef]
- Nayak, P.K.; Sendner, M.; Wenger, B.; Wang, Z.; Sharma, K.; Ramadan, A.J.; Lovrinčić, R.; Pucci, A.; Madhu, P.K.; Snaith, H.J. Impact of Bi3+ Heterovalent doping in organic-inorganic metal halide perovskite crystals. J. Am. Chem. Soc. 2018, 140, 574–577. [Google Scholar] [CrossRef]
- Wang, J.T.W.; Wang, Z.; Pathak, S.; Zhang, W.; Dequilettes, D.W.; Wisnivesky-Rocca-Rivarola, F.; Huang, J.; Nayak, P.K.; Patel, J.B.; Mohd Yusof, H.A.; et al. Efficient perovskite solar cells by metal ion doping. Energy Environ. Sci. 2016, 9, 2892–2901. [Google Scholar] [CrossRef]
- Miao, L.; Sun, P.; Cheng, F.; Shen, Y.; Wang, J.; Huang, W.; Chang, S.-Y.; Wang, M.; Zhu, B.; Shai, X.; et al. Achieving ordered and stable binary metal perovskite via strain engineering. Nano Energy 2018, 48, 117–127. [Google Scholar] [CrossRef]
- Muscarella, L.A.; Petrova, D.; Cervasio, R.J.; Farawar, A.; Lugier, O.; McLure, C.; Slaman, M.J.; Wang, J.; Ehrler, B.; von Hauff, E.; Williams, R.M. Air-stable and oriented mixed lead halide perovskite (FA/MA) by one-step deposition method using zinc iodide and alkylammonium additive. ACS Appl. Mater. Interfaces 2019. Accepted Manuscript. [Google Scholar] [CrossRef]
- Jiang, Q.; Zhao, Y.; Zhang, X.; Yang, X.; Chen, Y.; Chu, Z.; Ye, Q.; Li, X.; Yin, Z.; You, J. Surface passivation of perovskite film for efficient solar cells. Nat. Photonics 2019. [Google Scholar] [CrossRef]
- Yang, W.S.; Park, B.-W.; Jung, E.H.; Jeon, N.J.; Kim, Y.C.; Lee, D.U.; Shin, S.S.; Seo, J.; Kim, E.K.; Noh, J.H.; et al. Iodide management in formamidinium-lead-halide–based perovskite layers for efficient solar cells. Science 2017, 356, 1376–1379. [Google Scholar] [CrossRef]
- Yang, W.S.; Noh, J.H.; Jeon, N.J.; Kim, Y.C.; Ryu, S.; Seo, J.; Seok, S.I. High- performance photovoltaic perovskite layers fabricated through intramolecular exchange. Science 2015, 348, 1234–1237. [Google Scholar] [CrossRef]
- Kojima, A.; Teshima, K.; Shirai, Y.; Miyasaka, T. Organometal halide perovskites as visible-light sensitizers for photovoltaic cells. J. Am. Chem. Soc. 2009, 131, 6050–6051. [Google Scholar] [CrossRef]
- Shao, S.; Liu, J.; Portale, G.; Fang, H.-H.; Blake, G.R.; Brink, G.H.; Koster, L.A.; Loi, M.A. Highly reproducible SN-based hybrid perovskite solar cells with 9% efficiency. Adv. Energy Mater. 2018, 8, 1702019. [Google Scholar] [CrossRef]
- Soleimanioun, N.; Rani, M.; Sharma, S.; Kumar, A.; Tripathi, S.K. Binary metal zinc-lead perovskite built-in air ambient: Towards lead-less and stable perovskite materials. Sol. Energy Mater. Sol. Cells 2019, 191, 339–344. [Google Scholar] [CrossRef]









| Material | x (Zn) | τ (ns) Undoped (x = 0) | τ (ns) Doped | Reference |
|---|---|---|---|---|
| CsPb1-xZnxBr3 | <0.1 | 17 | 15 | [10] 1 |
| MAPb1-xZnxI3-[Cl−] | 0.03 | 74 | 154 | [11] |
| MAPb1-xZnxI3-[Cl−] | 0.001 | 10,740 | 12,910 | [13] 2 |
| MAPb1-xZnxI3 | 0.03 | 98 | 118 | [14] |
| MAPb1-xZnxI3 | 0.05 | 43 | 165 | [15] 3 |
| MAPb1-xZnxI3 | 0.10 | 43 | 247 | [15] 3 |
| MAPb1-xZnxI3 | 0.15 | 43 | 548 | [15] 3 |
| FA0.85MA0.15Pb1-xZnxI2.85Br0.15-[CPACl] | 0.025 | 7 | 12 | [16] 4 |
| FA0.85MA0.15Pb1-xZnxI2.85Br0.15-[CPACl] | 0.025 | - | 3.2; 8.5 | [16] 5 |
| MAPb1-xZnxI3-[Cl−] | 0.01 | 208 | 248 | [23] |
| FA0.85MA0.15Pb1-xZnxI2.85Br0.15 | 0.025 | 589 | 635 | [24] |
| FA0.85MA0.15Pb1-xZnxI2.85Br0.15-[CPACl] | 0.025 | - | 1548 | [24] |
| FA0.92MA0.08PbI3-[Cl−] | - | 364 | - | [25] |
| FA0.92MA0.08PbI3-[PEAI, Cl−] | - | 2835 | - | [25] |
| FA0.95MA0.05PbI2.85Br0.15 | - | 228 | - | [26] |
| FA0.95MA0.05PbI2.85Br0.15-[(I3)−] | - | 1105 | - | [26] |
| Material | x (Zn) | PCE (%) Undoped | PCE (%) Zn-Doped | d/ud | Reference |
|---|---|---|---|---|---|
| MAPb1-xZnxI3-[OAc−] | 0.016 | 7.27 | 9.69 | 1.33 | [9] 1 |
| MAPb1-xZnxI3-[Cl−] | 0.03 | 16.40 | 18.20 | 1.11 | [11] |
| MAPb1-xZnxI3-[Cl−] | 0.001 | 12.30 | 16.30 | 1.33 | [13] |
| MAPb1-xZnxI3 | 0.03 | 17.13 | 18.35 | 1.07 | [14] |
| MAPb1-xZnxI3 | 0.05 | 17.11 | 18.25 | 1.07 | [15] |
| MAPb1-xZnxI3-[Cl−] | 0.01 | 17.94 | 20.06 | 1.12 | [23] |
| FA0.92MA0.08PbI3-[PEAI, Cl−] | - | 23.32 | - | - | [25] 2 |
| FA0.95MA0.05PbI2.85Br0.15-[(I3)−] | - | 22.10 | - | - | [26] 3 |
| FA0.95MA0.05 PbI2.85 Br0.15 | - | 20.11 | - | - | [27] 4 |
| FA0.85MA0.15 PbI2.55 Br0.45 | - | 17.91 | - | - | [5] 5 |
| MAPbI3 | - | 3.81 | - | - | [28] 6 |
| FASnI3-[PEAI] | - | 9.00 | - | - | [29] |
| MASnIBr2 | - | 5.75 | - | - | [8] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kooijman, A.; Muscarella, L.A.; Williams, R.M. Perovskite Thin Film Materials Stabilized and Enhanced by Zinc(II) Doping. Appl. Sci. 2019, 9, 1678. https://doi.org/10.3390/app9081678
Kooijman A, Muscarella LA, Williams RM. Perovskite Thin Film Materials Stabilized and Enhanced by Zinc(II) Doping. Applied Sciences. 2019; 9(8):1678. https://doi.org/10.3390/app9081678
Chicago/Turabian StyleKooijman, Arjaan, Loreta A. Muscarella, and René M. Williams. 2019. "Perovskite Thin Film Materials Stabilized and Enhanced by Zinc(II) Doping" Applied Sciences 9, no. 8: 1678. https://doi.org/10.3390/app9081678