808-Nm Near-Infrared Laser Photobiomodulation versus Switched-Off Laser Placebo in Major Aphthae Management: A Randomized Double-Blind Controlled Trial
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Randomization and Masking
2.3. Procedures
2.4. Outcomes
2.5. Statistical Analysis
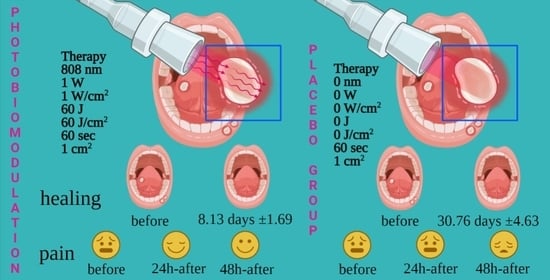
3. Results
3.1. Participants and Randomization
3.2. Primary Variable
3.3. Secondary Variable
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bilodeau, E.A.; Lalla, R.V. Recurrent oral ulceration: Etiology, classification, management, and diagnostic algorithm. Periodontol. 2000 2019, 80, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Akintoye, S.O.; Greenberg, M.S. Recurrent aphthous stomatitis. Dent. Clin. N. Am. 2014, 58, 281–297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rogers, R.S., 3rd. Recurrent aphthous stomatitis: Clinical characteristics and associated systemic disorders. Semin. Cutan. Med. Surg. 1997, 16, 278–283. [Google Scholar] [CrossRef]
- Rivera, C. Essentials of recurrent aphthous stomatitis. Biomed. Rep. 2019, 11, 47–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olejnik, M.; Ślebioda, Z.; Dorocka-Bobkowska, B. Low-level laser therapy (LLLT) in the treatment of recurrent aphthous stomatitis (RAS)—A promising treatment option: A report of two cases. Dent. Med Probl. 2019, 56, 317–321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Staines, K.; Greenwood, M. Aphthous ulcers (recurrent). BMJ Clin. Evid. 2015, 2015, 1303. [Google Scholar]
- Williams, L.A.; Bramwell, B.L. Current concepts in the treatment of recurrent aphthous ulcers. Int. J. Pharm. Compd. 2011, 15, 13–18. [Google Scholar]
- Zhang, Y.; Ng, K.-H.; Kuo, C.-Y.; Wu, D.-J. Chinese herbal medicine for recurrent aphthous stomatitis: A protocol for systematic review and meta-analysis. Medicine 2018, 97, e13681. [Google Scholar] [CrossRef]
- Mangal, B.; Sugandhi, A.; Kumathalli, K.I.; Sridhar, R. Alternative Medicine in Periodontal Therapy—A Review. J. Acupunct. Meridian Stud. 2012, 5, 51–56. [Google Scholar] [CrossRef] [Green Version]
- Janicki, M.P. Recurrent Herpes Labialis and Recurrent Aphthous Ulcerations: Psychological Components. Psychother. Psychosom. 1971, 19, 288–294. [Google Scholar] [CrossRef]
- Dos Santos, J.A.; Normando, A.G.C.; De Toledo, I.P.; Melo, G.; Canto, G.D.L.; Santos-Silva, A.R.; Guerra, E.N.S. Laser therapy for recurrent aphthous stomatitis: An overview. Clin. Oral Investig. 2019, 24, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Hanna, R.; Dalvi, S.; Benedicenti, S.; Amaroli, A.; Sălăgean, T.; Pop, I.D.; Todea, D.; Bordea, I.R.; Amaroli, A. Photobiomodulation Therapy in Oral Mucositis and Potentially Malignant Oral Lesions: A Therapy Towards the Future. Cancers 2020, 12, 1949. [Google Scholar] [CrossRef] [PubMed]
- Piotrowska, A.; Wierzbicka, J.; Żmijewski, M.A. Vitamin D in the skin physiology and pathology. Acta Biochim. Pol. 2016, 63, 17–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Werner, J.S.; Peterzell, D.H.; Scheetz, A.J. Light, Vision, and Aging. Optom. Vis. Sci. 1990, 67, 214–229. [Google Scholar] [CrossRef]
- Amaroli, A.; Pasquale, C.; Zekiy, A.; Utyuzh, A.; Benedicenti, S.; Signore, A.; Ravera, S. Photobiomodulation and Oxidative Stress: 980 nm Diode Laser Light Regulates Mitochondrial Activity and Reactive Oxygen Species Production. Oxid. Med. Cell. Longev. 2021, 2021, 6626286. [Google Scholar] [CrossRef] [PubMed]
- Ravera, S.; Colombo, E.; Pasquale, C.; Benedicenti, S.; Solimei, L.; Signore, A.; Amaroli, A. Mitochondrial Bioenergetic, Photobiomodulation and Trigeminal Branches Nerve Damage, What’s the Connection? A Review. Int. J. Mol. Sci. 2021, 22, 4347. [Google Scholar] [CrossRef]
- Amaroli, A.; Ferrando, S.; Benedicenti, S. Photobiomodulation Affects Key Cellular Pathways of all Life-Forms: Considerations on Old and New Laser Light Targets and the Calcium Issue. Photochem. Photobiol. 2019, 95, 455–459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colombo, E.; Signore, A.; Aicardi, S.; Zekiy, A.; Utyuzh, A.; Benedicenti, S.; Amaroli, A. Experimental and Clinical Ap-plications of Red and Near-Infrared Photobiomodulation on Endothelial Dysfunction: A Review. Biomedicines 2021, 9, 274. [Google Scholar] [CrossRef]
- Tunér, J.; Jenkins, P.A. Parameter Reproducibility in Photobiomodulation. Photomed. Laser Surg. 2016, 34, 91–92. [Google Scholar] [CrossRef] [Green Version]
- Albrektson, M.; Hedström, L.; Bergh, H. Recurrent aphthous stomatitis and pain management with low-level laser therapy: A randomized controlled trial. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2014, 117, 590–594. [Google Scholar] [CrossRef]
- Anand, V.; Gulati, M.; Govila, V.; Anand, B. Low level laser therapy in the treatment of aphthous ulcer. Indian J. Dent. Res. 2013, 24, 267–270. [Google Scholar] [CrossRef] [PubMed]
- Babu, B.; Uppada, U.; Tarakji, B.; Hussain, K.; Azzeghaibi, S.; Alzoghaibi, I. Versatility of diode lasers in low-level laser therapy for the management of recurrent aphthous stomatitis. J. Orofac. Sci. 2015, 7, 49. [Google Scholar] [CrossRef]
- Bladowski, M.; Konarska-Choroszucha, H.; Choroszucha, T. Comparison of Treatment Results of Recurrent Aphthous Stomatitis (RAS) with Low-and High-power Laser Irradiation vs. a Pharmaceutical Method (5-year Study). J. Oral Laser Appl. 2004, 4, 191–209. [Google Scholar]
- De Souza, T.O.F.; Martins, M.A.T.; Bussadori, S.K.; Fernandes, K.P.S.; Tanji, E.Y.; Mesquita-Ferrari, R.A.; Martins, M.D. Clinical evaluation of low-level laser treatment for recurring aphthous stomatitis. Photomed. Laser Surg. 2010, 28, S-85–S-88. [Google Scholar] [CrossRef]
- Jijin, M.J.; Rakaraddi, M.; Pai, J.; Jaishankar, H.P.; Krupashankar, R.; Kavitha, A.P.; Anjana, R.; Shobha, R. Low-level laser therapy versus 5% amlexanox: A comparison of treatment effects in a cohort of patients with minor aphthous ulcers. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2016, 121, 269–273. [Google Scholar] [CrossRef] [PubMed]
- Lalabonova, H.; Daskalov, H. Clinical assessment of the therapeutic effect of low-level laser therapy on chronic recurrent aphthous stomatitis. Biotechnol. Biotechnol. Equip. 2014, 28, 929–933. [Google Scholar] [CrossRef] [PubMed]
- Rocca, J.P.; Zhao, M.; Fornaini, C.; Tan, L.; Zhao, Z.; Merigo, E. Effect of laser irradiation on aphthae pain management: A four different wavelengths comparison. J. Photochem. Photobiol. B Biol. 2018, 189, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Soliman, H.A.; Mostafaa, D. Clinical Evaluation of 660 nm Diode Laser Therapy on the Pain, Size and Functional Disorders of Recurrent Aphthous Stomatitis. Open Access Maced. J. Med Sci. 2019, 7, 1516–1522. [Google Scholar] [CrossRef] [Green Version]
- Tezel, A.; Kara, C.; Balkaya, V.; Orbak, R. An Evaluation of Different Treatments for Recurrent Aphthous Stomatitis and Patient Perceptions: Nd:YAG Laser versus Medication. Photomed. Laser Surg. 2009, 27, 101–106. [Google Scholar] [CrossRef]
- Sanchez, P.M.; Femenias, J.C.; Tunér, J. Treatment of Aphthous Stomatitis Using Low Level Laser Therapy. Laser 2013, 3, 18–20. [Google Scholar]
- Yilmaz, H.G.; Albaba, M.R.; Caygur, A.; Cengiz, E.; Boke-Karacaoglu, F.; Tumer, H. Treatment of recurrent aphthous stomatitis with Er,Cr:YSGG laser irradiation: A randomized controlled split mouth clinical study. J. Photochem. Photobiol. B Biol. 2017, 170, 1–5. [Google Scholar] [CrossRef]
- Amaroli, A.; Ravera, S.; Parker, S.; Panfoli, I.; Benedicenti, A.; Benedicenti, S. An 808-nm Diode Laser with a Flat-Top Handpiece Positively Photobiomodulates Mitochondria Activities. Photomed. Laser Surg. 2016, 34, 564–571. [Google Scholar] [CrossRef]
- Amaroli, A.; Ravera, S.; Parker, S.; Panfoli, I.; Benedicenti, A.; Benedicenti, S. 808-nm laser therapy with a flat-top hand-piece photobiomodulates mitochondria activities of Paramecium primaurelia (Protozoa). Lasers Med. Sci. 2016, 31, 741–747. [Google Scholar] [CrossRef] [PubMed]
- Amaroli, A.; Ravera, S.; Parker, S.; Panfoli, I.; Benedicenti, A.; Benedicenti, S. Effect of 808 nm Diode Laser on Swimming Behavior, Food Vacuole Formation and Endogenous ATP Production of Paramecium primaurelia (Protozoa). Photochem. Photobiol. 2015, 91, 1150–1155. [Google Scholar] [CrossRef]
- Amaroli, A.; Ferrando, S.; Hanna, R.; Gallus, L.; Benedicenti, A.; Scarfì, S.; Pozzolini, M.; Benedicenti, S. The photobio-modulation effect of higher-fluence 808-nm laser therapy with a flat-top handpiece on the wound healing of the earth-worm Dendrobaena veneta: A brief report. Lasers Med. Sci. 2018, 33, 221–225. [Google Scholar] [CrossRef] [PubMed]
- Amaroli, A.; Ravera, S.; Baldini, F.; Benedicenti, S.; Panfoli, I.; Vergani, L. Photobiomodulation with 808-nm diode laser light promotes wound healing of human endothelial cells through increased reactive oxygen species production stimulating mitochondrial oxidative phosphorylation. Lasers Med. Sci. 2019, 34, 495–504. [Google Scholar] [CrossRef] [PubMed]
- Amaroli, A.; Sabbieti, M.G.; Marchetti, L.; Zekiy, A.O.; Utyuzh, A.S.; Marchegiani, A.; Laus, F.; Cuteri, V.; Benedicenti, S.; Agas, D. The effects of 808-nm near-infrared laser light irradiation on actin cytoskeleton reorganization in bone marrow mesenchymal stem cells. Cell Tissue Res. 2021, 383, 1003–1016. [Google Scholar] [CrossRef] [PubMed]
- Amaroli, A.; Agas, D.; Laus, F.; Cuteri, V.; Hanna, R.; Sabbieti, M.G.; Benedicenti, S. The Effects of Photobiomodulation of 808 nm Diode Laser Therapy at Higher Fluence on the in Vitro Osteogenic Differentiation of Bone Marrow Stromal Cells. Front. Physiol. 2018, 9, 123. [Google Scholar] [CrossRef] [Green Version]
- Hanna, R.; Agas, D.; Benedicenti, S.; Ferrando, S.; Laus, F.; Cuteri, V.; Lacava, G.; Sabbieti, M.G.; Amaroli, A. A Comparative Study between the Effectiveness of 980 nm Photobiomodulation Delivered by Hand-Piece with Gaussian vs. Flat-Top Profiles on Osteoblasts Maturation. Front. Endocrinol. 2019, 10, 92. [Google Scholar] [CrossRef] [Green Version]
- Greer, R.O., Jr.; Lindenmuth, J.E.; Juarez, T.; Khandwala, A. A double-blind study of topically applied 5% amlexanox in the treatment of aphthous ulcers. J. Oral Maxillofac. Surg. 1993, 51, 243–248. [Google Scholar] [CrossRef]
- Selting, W. Atlas of Laser Therapy: State of the Art, 4th ed.; Teamwork Media Srl: Villa Carcina, Italy, 2016; pp. 225–236. [Google Scholar]
- Amaroli, A.; Benedicenti, A.; Ferrando, S.; Parker, S.; Selting, W.; Gallus, L.; Benedicenti, S. Photobiomodulation by Infrared Diode Laser: Effects on Intracellular Calcium Concentration and Nitric Oxide Production of Paramecium. Photochem. Photobiol. 2016, 92, 854–862. [Google Scholar] [CrossRef] [PubMed]
- Amaroli, A.; Ferrando, S.; Pozzolini, M.; Gallus, L.; Parker, S.; Benedicenti, S. The earthworm Dendrobaena veneta (Annelida): A new experimental-organism for photobiomodulation and wound healing. Eur. J. Histochem. 2018, 62, 2867. [Google Scholar] [CrossRef] [PubMed]
- Amaroli, A.; Parker, S.; Dorigo, G.; Benedicenti, A.; Benedicenti, S. Paramecium: A Promising Non-Animal Bioassay to Study the Effect of 808 nm Infrared Diode Laser Photobiomodulation. Photomed. Laser Surg. 2015, 33, 35–40. [Google Scholar] [CrossRef] [PubMed]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pasquale, C.; Colombo, E.; Benedicenti, S.; Signore, A.; Amaroli, A. 808-Nm Near-Infrared Laser Photobiomodulation versus Switched-Off Laser Placebo in Major Aphthae Management: A Randomized Double-Blind Controlled Trial. Appl. Sci. 2021, 11, 4717. https://doi.org/10.3390/app11114717
Pasquale C, Colombo E, Benedicenti S, Signore A, Amaroli A. 808-Nm Near-Infrared Laser Photobiomodulation versus Switched-Off Laser Placebo in Major Aphthae Management: A Randomized Double-Blind Controlled Trial. Applied Sciences. 2021; 11(11):4717. https://doi.org/10.3390/app11114717
Chicago/Turabian StylePasquale, Claudio, Esteban Colombo, Stefano Benedicenti, Antonio Signore, and Andrea Amaroli. 2021. "808-Nm Near-Infrared Laser Photobiomodulation versus Switched-Off Laser Placebo in Major Aphthae Management: A Randomized Double-Blind Controlled Trial" Applied Sciences 11, no. 11: 4717. https://doi.org/10.3390/app11114717