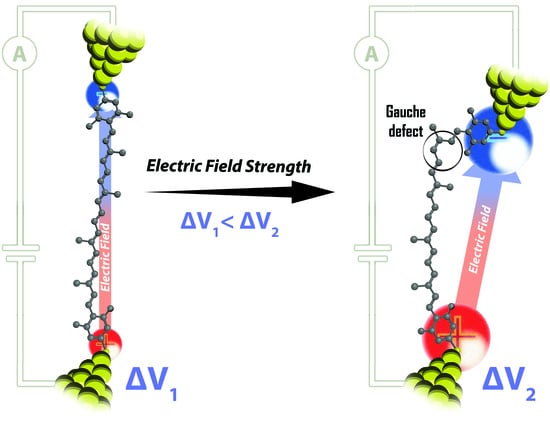
Tuning Single-Molecule Conductance by Controlled Electric Field-Induced trans-to-cis Isomerisation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experiments
2.2. Simulations
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
Appendix A.1. In Situ Irreversible High-Conductance (HC) to Low-Conductance (LC) Conversion

Appendix A.2. Energy Profile for trans-to-cis Rotation in the Absence of an Electric Field



| cis-TMC isomer: | |||||||||||
| GS | S1 | S2 | S3 | S4 | S5 | S6 | S7 | S8 | S9 | S10 | |
| GS | 0.9995 | 0.0004 | 0.0262 | −0.0148 | −0.0003 | −0.0046 | −0.0004 | −0.0002 | −0.0001 | −0.0003 | 0.0001 |
| S1 | −0.0112 | 0.8926 | 0.4447 | 0.0352 | 0.0272 | 0.0565 | 0.0076 | −0.0126 | 0.0002 | 0.0043 | 0.0044 |
| S2 | 0.0236 | 0.4445 | −0.8925 | 0.0177 | −0.0617 | 0.0302 | 0.0000 | 0.0173 | 0.0012 | 0.0012 | −0.0063 |
| S3 | −0.0140 | 0.0333 | 0.0324 | −0.8770 | −0.4738 | 0.0048 | −0.0302 | 0.0462 | −0.0019 | −0.0091 | 0.0282 |
| S4 | 0.0053 | −0.0230 | 0.0590 | 0.4740 | −0.8741 | 0.0295 | −0.0677 | −0.0331 | −0.0067 | −0.0221 | −0.0137 |
| S5 | 0.0042 | −0.0623 | −0.0051 | −0.0230 | 0.0285 | 0.9713 | 0.0093 | −0.2210 | 0.0008 | −0.0008 | 0.0487 |
| S6 | −0.0003 | 0.0046 | −0.0029 | −0.0077 | 0.0506 | −0.0064 | −0.7369 | −0.0596 | −0.5787 | 0.3402 | −0.0127 |
| S7 | −0.0014 | 0.0138 | −0.0203 | −0.0528 | 0.0092 | −0.2141 | −0.0937 | −0.9482 | 0.1290 | −0.1580 | −0.0343 |
| S8 | −0.0001 | 0.0038 | 0.0041 | 0.0060 | 0.0582 | 0.0538 | −0.6394 | 0.2106 | 0.4624 | −0.5703 | −0.0387 |
| S9 | 0.0002 | −0.0023 | −0.0001 | 0.0049 | −0.0080 | −0.0081 | −0.1834 | −0.0161 | 0.6555 | 0.7209 | 0.1284 |
| S10 | −0.0002 | −0.0016 | 0.0081 | −0.0304 | −0.0037 | 0.0520 | 0.0145 | 0.0141 | 0.0701 | 0.1171 | −0.9886 |
| trans-TMC isomer: | |||||||||||
| GS | S1 | S2 | S3 | S4 | S5 | S6 | S7 | S8 | S9 | S10 | |
| GS | 0.9997 | −0.0013 | −0.0217 | −0.0123 | 0.0029 | −0.0052 | 0.0008 | −0.0013 | −0.0002 | 0.0009 | 0.0017 |
| S1 | 0.0101 | −0.8381 | 0.5414 | −0.0248 | −0.0201 | −0.0566 | 0.01 | −0.007 | 0.0017 | 0.0023 | −0.0008 |
| S2 | −0.0192 | −0.5417 | −0.8386 | −0.0041 | 0.0462 | −0.0205 | −0.0155 | 0.0062 | −0.0006 | −0.0016 | −0.0057 |
| S3 | 0.0106 | −0.0178 | 0.0302 | 0.9064 | 0.4184 | 0.0022 | −0.0217 | 0.0244 | −0.0037 | −0.0004 | 0.0314 |
| S4 | −0.007 | 0.0188 | 0.0404 | −0.4185 | 0.9042 | −0.0309 | 0.0375 | 0.0506 | −0.0018 | −0.0149 | −0.0064 |
| S5 | −0.0053 | 0.0568 | −0.0184 | 0.0222 | −0.0287 | −0.9782 | 0.189 | −0.0427 | −0.0001 | 0.0055 | 0.0241 |
| S6 | −0.0013 | 0.006 | 0.0045 | −0.019 | 0.0511 | −0.0663 | −0.5116 | −0.7544 | −0.3966 | −0.0344 | 0.0567 |
| S7 | −0.0002 | −0.0101 | −0.0151 | 0.0287 | 0.0066 | 0.1766 | 0.829 | −0.419 | −0.3031 | −0.0968 | −0.0596 |
| S8 | −0.0009 | 0.0002 | −0.0059 | 0.0014 | 0.0336 | 0.0388 | 0.0798 | −0.4876 | 0.8005 | 0.3317 | 0.0489 |
| S9 | −0.0016 | −0.0022 | −0.0036 | −0.0167 | 0.0005 | 0.0221 | 0.0652 | 0.1119 | −0.3153 | 0.8471 | 0.4066 |
| S10 | −0.0014 | −0.0049 | −0.0045 | −0.0245 | −0.0119 | 0.0293 | 0.0488 | −0.0036 | 0.1032 | −0.4018 | 0.9077 |
Appendix A.3. cis-TMC-syn (Synthetic) Carotenoid Control Experiments


Appendix A.4. Synthetic Details

References
- Nair, V.; Muñoz-Batista, M.J.; Fernández-García, M.; Luque, R.; Colmenares, J.C. Thermo-Photocatalysis: Environmental and Energy Applications. ChemSusChem 2019, 12, 2098–2116. [Google Scholar] [CrossRef]
- Shaik, S.; Danovich, D.; Joy, J.; Wang, Z.; Stuyver, T. Electric-Field Mediated Chemistry: Uncovering and Exploiting the Potential of (Oriented) Electric Fields to Exert Chemical Catalysis and Reaction Control. J. Am. Chem. Soc. 2020, 142, 12551–12562. [Google Scholar] [CrossRef]
- Tang, C.; Zheng, J.; Ye, Y.; Liu, J.; Chen, L.; Yan, Z.; Chen, Z.; Chen, L.; Huang, X.; Bai, J.; et al. Electric-Field-Induced Connectivity Switching in Single-Molecule Junctions. Science 2020, 23, 100770. [Google Scholar] [CrossRef] [Green Version]
- Gorin, C.F.; Beh, E.S.; Kanan, M.W. An Electric Field–Induced Change in the Selectivity of a Metal Oxide–Catalyzed Epoxide Rearrangement. J. Am. Chem. Soc. 2011, 134, 186–189. [Google Scholar] [CrossRef] [PubMed]
- Aragonès, A.C.; Haworth, N.L.; Darwish, N.; Ciampi, S.; Bloomfield, N.J.; Wallace, G.G.; Diez-Perez, I.; Coote, M.L. Electrostatic catalysis of a Diels–Alder reaction. Nat. Cell Biol. 2016, 531, 88–91. [Google Scholar] [CrossRef] [PubMed]
- Morgenstern, K. Isomerization Reactions on Single Adsorbed Molecules. Accounts Chem. Res. 2009, 42, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.S.; Ye, T.; Takami, T.; Yu, B.-C.; Flatt, A.K.; Tour, J.M.; Weiss, P.S. Reversible Photo-Switching of Single Azobenzene Molecules in Controlled Nanoscale Environments. Nano Lett. 2008, 8, 1644–1648. [Google Scholar] [CrossRef] [PubMed]
- Mativetsky, J.M.; Pace, G.; Elbing, M.; Rampi, M.A.; Mayor, M.; Samorì, P. Azobenzenes as Light-Controlled Molecular Electronic Switches in Nanoscale Metal−Molecule−Metal Junctions. J. Am. Chem. Soc. 2008, 130, 9192–9193. [Google Scholar] [CrossRef]
- Martin, S.; Haiss, W.; Higgins, S.J.; Nichols, R.J. The Impact of E–Z Photo-Isomerization on Single Molecular Conductance. Nano Lett. 2010, 10, 2019–2023. [Google Scholar] [CrossRef]
- Donhauser, Z.J. Conductance Switching in Single Molecules Through Conformational Changes. Science 2001, 292, 2303–2307. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, G.K.; Tomfohr, J.K.; Li, J.; Sankey, O.F.; Zarate, X.; Primak, A.; Terazono, Y.; Moore, T.A.; Moore, A.L.; Gust, D.; et al. Electron Transport Properties of a Carotene Molecule in a Metal−(Single Molecule)−Metal Junction. J. Phys. Chem. B 2003, 107, 6162–6169. [Google Scholar] [CrossRef]
- Venkataraman, L.; Klare, J.E.; Nuckolls, C.; Hybertsen, M.S.; Steigerwald, M.L. Dependence of single-molecule junction conductance on molecular conformation. Nat. Cell Biol. 2006, 442, 904–907. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tao, N.J. Electron transport in molecular junctions. Nat. Nanotechnol. 2006, 1, 173–181. [Google Scholar] [CrossRef]
- Cai, Z.-L.; Crossley, M.J.; Reimers, J.R.; Kobayashi, A.R.; Amos, R.D. Density Functional Theory for Charge Transfer: The Nature of the N-Bands of Porphyrins and Chlorophylls Revealed through CAM-B3LYP, CASPT2, and SAC-CI Calculations. J. Phys. Chem. B 2006, 110, 15624–15632. [Google Scholar] [CrossRef]
- Leary, E.; Roche, C.; Jiang, H.-W.; Grace, I.; González, M.T.; Rubio-Bollinger, G.; Romero-Muñiz, C.; Xiong, Y.; Al-Galiby, Q.; Noori, M.; et al. Detecting Mechanochemical Atropisomerization within an STM Break Junction. J. Am. Chem. Soc. 2018, 140, 710–718. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Tomfohr, J.K.; Sankey, O.F. Theoretical study of carotene as a molecular wire. Phys. E Low Dimens. Syst. Nanostructures 2003, 19, 133–138. [Google Scholar] [CrossRef]
- Del Valle, M.; Gutierrez, R.; Tejedor, C.; Cuniberti, G. Tuning the conductance of a molecular switch. Nat. Nanotechnol. 2007, 2, 176–179. [Google Scholar] [CrossRef] [Green Version]
- Dhivya, G.; Nagarajan, V.; Chandiramouli, R. First-principles studies on switching properties of azobenzene based molecular device. Chem. Phys. Lett. 2016, 660, 27–32. [Google Scholar] [CrossRef]
- Li, C.; Pobelov, I.; Wandlowski, T.; Bagrets, A.; Arnold, A.A.; Evers, F. Charge Transport in Single Au | Alkanedithiol | Au Junctions: Coordination Geometries and Conformational Degrees of Freedom. J. Am. Chem. Soc. 2008, 130, 318–326. [Google Scholar] [CrossRef] [Green Version]
- Cao, Y.; Dong, S.; Liu, S.; Liu, Z.; Guo, X. Toward Functional Molecular Devices Based on Graphene-Molecule Junctions. Angew. Chem. Int. Ed. 2013, 52, 3906–3910. [Google Scholar] [CrossRef]
- Sotthewes, K.; Geskin, V.; Heimbuch, R.; Kumar, A.; Zandvliet, H.J.W. Research Update: Molecular electronics: The single-molecule switch and transistor. APL Mater. 2014, 2, 10701. [Google Scholar] [CrossRef]
- Kim, M.; Jung, H.; Aragonès, A.C.; Diez-Perez, I.; Ahn, K.-H.; Chung, W.-J.; Kim, D.; Koo, S. Role of Ring Ortho Substituents on the Configuration of Carotenoid Polyene Chains. Org. Lett. 2018, 20, 493–496. [Google Scholar] [CrossRef]
- Schwarz, F.; Lörtscher, E. Break-junctions for investigating transport at the molecular scale. J. Phys. Condens. Matter 2014, 26, 474201. [Google Scholar] [CrossRef] [PubMed]
- Komoto, Y.; Fujii, S.; Iwane, M.; Kiguchi, M. Single-molecule junctions for molecular electronics. J. Mater. Chem. C 2016, 4, 8842–8858. [Google Scholar] [CrossRef]
- Stefani, D.; Perrin, M.; Gutiérrez-Cerón, C.; Aragonès, A.C.; Labra-Muñoz, J.; Carrasco, R.D.C.; Matsushita, Y.; Futera, Z.; Labuta, J.; Ngo, T.H.; et al. Mechanical Tuning of Through-Molecule Conductance in a Conjugated Calix[4]pyrrole. Chemitry 2018, 3, 6473–6478. [Google Scholar] [CrossRef]
- Aragonès, A.C.; Darwish, N.; Im, J.; Lim, B.; Choi, J.; Koo, S.; Díez-Pérez, I. Fine-Tuning of Single-Molecule Conductance by Tweaking Both Electronic Structure and Conformation of Side Substituents. Chem. A Eur. J. 2015, 21, 7716–7720. [Google Scholar] [CrossRef]
- Chen, F.; Tao, N.J. Electron Transport in Single Molecules: From Benzene to Graphene. Accounts Chem. Res. 2009, 42, 429–438. [Google Scholar] [CrossRef]
- Li, X.; He, J.; Hihath, J.; Xu, B.; Lindsay, S.M.; Tao, N. Conductance of Single Alkanedithiols: Conduction Mechanism and Effect of Molecule−Electrode Contacts. J. Am. Chem. Soc. 2006, 128, 2135–2141. [Google Scholar] [CrossRef]
- Kushmerick, J.G.; Holt, D.B.; Pollack, S.K.; Ratner, M.A.; Yang, J.C.; Schull, T.L.; Naciri, J.; Moore, M.H.; Shashidhar, R. Effect of Bond-Length Alternation in Molecular Wires. J. Am. Chem. Soc. 2002, 124, 10654–10655. [Google Scholar] [CrossRef] [PubMed]
- Tracewell, C.A.; Cua, A.; Stewart, D.H.; Bocian, D.F.; Brudvig, G.W. Characterization of Carotenoid and Chlorophyll Photooxidation in Photosystem II. Biochemitry 2001, 40, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Tracewell, C.A.; Brudvig, G.W. Multiple Redox-Active Chlorophylls in the Secondary Electron-Transfer Pathways of Oxygen-Evolving Photosystem II. Biochemitry 2008, 47, 11559–11572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garner, M.H.; Solomon, G.C. Simultaneous Suppression of π- and σ-Transmission in π-Conjugated Molecules. J. Phys. Chem. Lett. 2020, 11, 7400–7406. [Google Scholar] [CrossRef] [PubMed]
- Hunter, J.D. Matplotlib: A 2D Graphics Environment. Comput. Sci. Eng. 2007, 9, 90–95. [Google Scholar] [CrossRef]
- Neese, F. Software update: The ORCA program system, version 4.0. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2018, 8, 8. [Google Scholar] [CrossRef]
- Runge, E.; Gross, E.K.U. Density-Functional Theory for Time-Dependent Systems. Phys. Rev. Lett. 1984, 52, 997–1000. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A 1988, 38, 3098–3100. [Google Scholar] [CrossRef]
- Perdew, J.P. Density-functional approximation for the correlation energy of the inhomogeneous electron gas. Phys. Rev. B 1986, 33, 8822–8824. [Google Scholar] [CrossRef]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef]
- Weigend, F. Accurate Coulomb-fitting basis sets for H to Rn. Phys. Chem. Chem. Phys. 2006, 8, 1057–1065. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef] [Green Version]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef]
- Plasser, F.; Ruckenbauer, M.; Mai, S.; Oppel, M.; Marquetand, P.; González, L. Efficient and Flexible Computation of Many-Electron Wave Function Overlaps. J. Chem. Theory Comput. 2016, 12, 1207–1219. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Li, X.; Hihath, J.; Huang, Z.; Tao, N. Effect of Anchoring Groups on Single-Molecule Conductance: Comparative Study of Thiol-, Amine-, and Carboxylic-Acid-Terminated Molecules. J. Am. Chem. Soc. 2006, 128, 15874–15881. [Google Scholar] [CrossRef] [PubMed]
- Haiss, W.; Nichols, R.J.; Van Zalinge, H.; Higgins, S.J.; Bethell, D.; Schiffrin, D.J. Measurement of single molecule conductivity using the spontaneous formation of molecular wires. Phys. Chem. Chem. Phys. 2004, 6, 4330–4337. [Google Scholar] [CrossRef]
- Pla-Vilanova, P.; Aragonès, A.C.; Ciampi, S.; Sanz, F.; Darwish, N.; Diez-Perez, I. The spontaneous formation of single-molecule junctions via terminal alkynes. Nanotechnology 2015, 26, 381001. [Google Scholar] [CrossRef] [PubMed]
- Aragonès, A.C.; Darwish, N.; Ciampi, S.; Sanz, F.; Gooding, J.J.; Díez-Pérez, I. Single-molecule electrical contacts on silicon electrodes under ambient conditions. Nat. Commun. 2017, 8, 15056. [Google Scholar] [CrossRef]
- Xu, B. Measurement of Single-Molecule Resistance by Repeated Formation of Molecular Junctions. Science 2003, 301, 1221–1223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Z.; Han, B.; Meszaros, G.; Pobelov, I.; Wandlowski, T.; Błaszczyk, A.; Mayor, M. Two-dimensional assembly and local redox-activity of molecular hybrid structures in an electrochemical environment. Faraday Discuss. 2005, 131, 121–143. [Google Scholar] [CrossRef] [Green Version]
- Bruot, C.; Hihath, J.; Tao, N. Mechanically controlled molecular orbital alignment in single molecule junctions. Nat. Nanotechnol. 2011, 7, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Inatomi, J.; Fujii, S.; Marqués-González, S.; Masai, H.; Tsuji, Y.; Terao, J.; Kiguchi, M. Effect of Mechanical Strain on Electric Conductance of Molecular Junctions. J. Phys. Chem. C 2015, 119, 19452–19457. [Google Scholar] [CrossRef]
- Qi, J.; Gao, Y.; Jia, H.; Richter, M.; Huang, L.; Cao, Y.; Yang, H.; Zheng, Q.; Berger, R.; Liu, J.; et al. Force-Activated Isomerization of a Single Molecule. J. Am. Chem. Soc. 2020, 142, 10673–10680. [Google Scholar] [CrossRef]
- Alemani, M.; Peters, M.V.; Hecht, S.; Rieder, K.-H.; Moresco, A.F.; Grill, L. Electric Field-Induced Isomerization of Azobenzene by STM. J. Am. Chem. Soc. 2006, 128, 14446–14447. [Google Scholar] [CrossRef] [PubMed]
- Zang, Y.; Zou, Q.; Fu, T.; Ng, F.; Fowler, B.; Yang, J.; Li, H.; Steigerwald, M.L.; Nuckolls, C.; Venkataraman, L. Directing isomerization reactions of cumulenes with electric fields. Nat. Commun. 2019, 10, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Chen, F.; D’Agosta, R.; Bennett, P.A.; Di Ventra, M.; Tao, N. Local ionic and electron heating in single-molecule junctions. Nat. Nanotechnol. 2007, 2, 698–703. [Google Scholar] [CrossRef] [PubMed]
- Blum, A.S.; Kushmerick, J.G.; Long, D.P.; Patterson, C.H.; Yang, J.C.; Henderson, J.C.; Yao, Y.; Tour, J.M.; Shashidhar, R.; Ratna, B.R. Molecularly inherent voltage-controlled conductance switching. Nat. Mater. 2005, 4, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Diez-Perez, I.; Hihath, J.; Hines, T.; Wang, Z.-S.; Zhou, G.; Müllen, K.; Tao, N. Controlling single-molecule conductance through lateral coupling of π orbitals. Nat. Nanotechnol. 2011, 6, 226–231. [Google Scholar] [CrossRef]
- Kitaguchi, Y.; Habuka, S.; Okuyama, H.; Hatta, S.; Aruga, T.; Frederiksen, T.; Paulsson, M.; Ueba, H. Controlling single-molecule junction conductance by molecular interactions. Sci. Rep. 2015, 5, 11796. [Google Scholar] [CrossRef] [Green Version]
- Yoshida, K.; Pobelov, I.V.; Manrique, D.Z.; Pope, T.; Mészáros, G.; Gulcur, M.; Bryce, M.R.; Lambert, C.J.; Wandlowski, T. Correlation of breaking forces, conductances and geometries of molecular junctions. Sci. Rep. 2015, 5, srep09002. [Google Scholar] [CrossRef] [Green Version]
- Reckien, W.; Eggers, M.; Bredow, T. Theoretical study of the adsorption of benzene on coinage metals. Beilstein J. Org. Chem. 2014, 10, 1775–1784. [Google Scholar] [CrossRef] [Green Version]
- Meisner, J.S.; Ahn, S.; Aradhya, S.V.; Krikorian, M.; Parameswaran, R.; Steigerwald, M.; Venkataraman, L.; Nuckolls, C. Importance of Direct Metal−π Coupling in Electronic Transport Through Conjugated Single-Molecule Junctions. J. Am. Chem. Soc. 2012, 134, 20440–20445. [Google Scholar] [CrossRef]
- Roke, D.; Wezenberg, S.J.; Feringa, B.L. Molecular rotary motors: Unidirectional motion around double bonds. Proc. Natl. Acad. Sci. USA 2018, 115, 9423–9431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hall, C.R.; Conyard, J.; Heisler, I.A.; Jones, G.A.; Frost, J.; Browne, W.R.; Feringa, B.L.; Meech, S.R. Ultrafast Dynamics in Light-Driven Molecular Rotary Motors Probed by Femtosecond Stimulated Raman Spectroscopy. J. Am. Chem. Soc. 2017, 139, 7408–7414. [Google Scholar] [CrossRef] [Green Version]
- Pang, X.; Cui, X.; Hu, D.; Jiang, C.; Zhao, D.; Lan, Z.; Li, F. “Watching” the Dark State in Ultrafast Nonadiabatic Photoisomerization Process of a Light-Driven Molecular Rotary Motor. J. Phys. Chem. A 2017, 121, 1240–1249. [Google Scholar] [CrossRef] [PubMed]
- Martin, R.L. Natural transition orbitals. J. Chem. Phys. 2003, 118, 4775–4777. [Google Scholar] [CrossRef]
- Kazaryan, A.; Lan, Z.; Schäfer, L.V.; Thiel, W.; Filatov, M. Surface Hopping Excited-State Dynamics Study of the Photoisomerization of a Light-Driven Fluorene Molecular Rotary Motor. J. Chem. Theory Comput. 2011, 7, 2189–2199. [Google Scholar] [CrossRef] [PubMed] [Green Version]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quintans, C.S.; Andrienko, D.; Domke, K.F.; Aravena, D.; Koo, S.; Díez-Pérez, I.; Aragonès, A.C. Tuning Single-Molecule Conductance by Controlled Electric Field-Induced trans-to-cis Isomerisation. Appl. Sci. 2021, 11, 3317. https://doi.org/10.3390/app11083317
Quintans CS, Andrienko D, Domke KF, Aravena D, Koo S, Díez-Pérez I, Aragonès AC. Tuning Single-Molecule Conductance by Controlled Electric Field-Induced trans-to-cis Isomerisation. Applied Sciences. 2021; 11(8):3317. https://doi.org/10.3390/app11083317
Chicago/Turabian StyleQuintans, C.S., Denis Andrienko, Katrin F. Domke, Daniel Aravena, Sangho Koo, Ismael Díez-Pérez, and Albert C. Aragonès. 2021. "Tuning Single-Molecule Conductance by Controlled Electric Field-Induced trans-to-cis Isomerisation" Applied Sciences 11, no. 8: 3317. https://doi.org/10.3390/app11083317