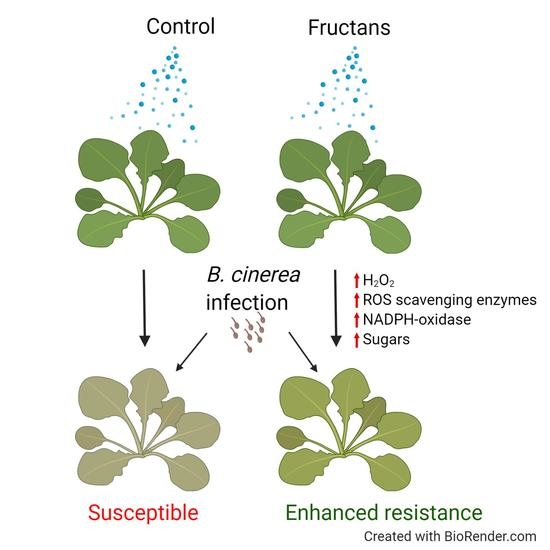
Fructans Prime ROS Dynamics and Botrytis cinerea Resistance in Arabidopsis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Growth Conditions
2.2. B. cinerea Cultivation and Preparation
2.3. Preparation of Priming Compounds
2.4. Plant Treatments
2.5. B. cinerea Infection and Disease Scoring
2.6. ROS Burst Measurements
2.7. H2O2 Extraction and Quantification
2.8. Antioxidant Enzyme Extraction and Activity Measurements
2.9. Soluble Sugar Extraction and Quantification
2.10. Graphical Preperation and Statistical Analysis
3. Results
3.1. Inulin- and Levan-Type Fructans Induce Resistance against B. cinerea
3.2. B. cinerea Germination and Growth Is Not Significantly Affected by Fructans
3.3. Inulin and LOS Prime the flg22 and OGs Elicitor-Mediated ROS Burst
3.4. Inulin and LOS Induce ROS Accumulation and ROS-Scavenging Enzymes
3.5. Fructan Treatments Prevent Fluctuating Sugar Levels During Infection
4. Discussion
5. Conclusions and Perspectives
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| APX | Ascorbate peroxidase |
| BABA | β-Aminobutyric acid |
| BFO | Burdock fructooligosaccharide |
| CAT | Catalase |
| DAB | 3,3′-Diaminobenzidine |
| DAMP | Damage Associated Molecular Pattern |
| DPI | Diphenyleneiodonium |
| Fru | Fructose |
| GABA | Gamma aminobutyric acid |
| Glc | Glucose |
| GPX | Guaiacol peroxidase |
| IC | Infection control |
| LOS | Levan oligosaccharide |
| MAMP | Microbe Associated Molecular Pattern |
| OG | Oligogalacturonides |
| PDA | Potato dextrose agar |
| RLU | Relative Light Units |
| ROS | Reactive oxygen species |
| SA | Salicylic acid |
| Suc | Sucrose |
References
- Rasmann, S.; de Vos, M.; Jander, G. Ecological role of transgenerational resistance against biotic threats. Plant Signal. Behav. 2012, 7, 447–449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wittstock, U.; Gershenzon, J. Constitutive plant toxins and their role in defense against herbivores and pathogens. Curr. Opin. Plant Biol. 2002, 5, 300–307. [Google Scholar] [CrossRef]
- Frost, C.J.; Mescher, M.C.; Carlson, J.E.; De Moraes, C.M. Plant defense priming against herbivores: Getting ready for a different battle. Plant Physiol. 2008, 146, 818–824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moreira, X.; Zas, R.; Sampedro, L. Differential allocation of constitutive and induced chemical defenses in pine tree juveniles: A test of the optimal defense theory. PLoS ONE 2012, 7, e34006. [Google Scholar] [CrossRef] [Green Version]
- Dubreuil-Maurizi, C.; Trouvelot, S.; Frettinger, P.; Pugin, A.; Wendehenne, D.; Poinssot, B. β-Aminobutyric acid primes an NADPH oxidase-dependent reactive oxygen species production during grapevine-triggered immunity. Mol. Plant Microbe Interact. 2010, 23, 1012–1021. [Google Scholar] [CrossRef]
- Bindschedler, L.V.; Minibayeva, F.; Gardner, S.L.; Gerrish, C.; Davies, D.R.; Bolwell, G.P. Early signalling events in the apoplastic oxidative burst in suspension cultured French bean cells involve cAMP and Ca2+. New Phytol. 2001, 151, 185–194. [Google Scholar] [CrossRef]
- Hua, L.; Yong, C.; Zhanquan, Z.; Boqiang, L.; Guozheng, Q.; Shiping, T. Pathogenic mechanisms and control strategies of Botrytis cinerea causing post-harvest decay in fruits and vegetables. Food Qual. Saf. 2018, 2, 111–119. [Google Scholar] [CrossRef] [Green Version]
- Torres, M.A.; Morales, J.; Sánchez-Rodríguez, C.; Molina, A.; Dangl, J.L. Functional interplay between Arabidopsis NADPH oxidases and heterotrimeric G protein. Mol. Plant-Microbe Interact. 2013, 26, 686–694. [Google Scholar] [CrossRef] [Green Version]
- Pogány, M.; von Rad, U.; Grün, S.; Dongó, A.; Pintye, A.; Simoneau, P.; Bahnweg, G.; Kiss, L.; Barna, B.; Durner, J. Dual roles of reactive oxygen species and NADPH oxidase RBOHD in an Arabidopsis-Alternaria pathosystem. Plant Physiol. 2009, 151, 1459–1475. [Google Scholar] [CrossRef] [Green Version]
- Conrath, U. Priming of induced plant defense responses. Adv. Bot. Res. 2009, 51, 361–395. [Google Scholar]
- Aranega-Bou, P.; de la O Leyva, M.; Finiti, I.; García-Agustín, P.; González-Bosch, C. Priming of plant resistance by natural compounds. Hexanoic acid as a model. Front. Plant Sci. 2014, 5, 488. [Google Scholar] [CrossRef] [Green Version]
- Martinez-Medina, A.; Flors, V.; Heil, M.; Mauch-Mani, B.; Pieterse, C.M.J.; Pozo, M.J.; Ton, J.; van Dam, N.M.; Conrath, U. Recognizing plant defense priming. Trends Plant Sci. 2016, 21, 818–822. [Google Scholar] [CrossRef] [Green Version]
- Ferrari, S.; Galletti, R.; Denoux, C.; De Lorenzo, G.; Ausubel, F.M.; Dewdney, J. Resistance to Botrytis cinerea induced in Arabidopsis by elicitors is independent of salicylic acid, ethylene, or jasmonate signaling but requires PHYTOALEXIN DEFICIENT3. Plant Physiol. 2007, 144, 367–379. [Google Scholar] [CrossRef] [Green Version]
- Thevenet, D.; Pastor, V.; Baccelli, I.; Balmer, A.; Vallat, A.; Neier, R.; Glauser, G.; Mauch-Mani, B. The priming molecule β-aminobutyric acid is naturally present in plants and is induced by stress. New Phytol. 2017, 213, 552–559. [Google Scholar] [CrossRef]
- Felix, G.; Duran, J.D.; Volko, S.; Boller, T. Plants have a sensitive perception system for the most conserved domain of bacterial flagellin. Plant J. 1999, 18, 265–276. [Google Scholar] [CrossRef]
- Ferrari, S.; Savatin, D.V.; Sicilia, F.; Gramegna, G.; Cervone, F.; De Lorenzo, G. Oligogalacturonides: Plant damage-associated molecular patterns and regulators of growth and development. Front. Plant Sci. 2013, 4, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Chaliha, C.; Rugen, M.D.; Field, R.A.; Kalita, E. Glycans as modulators of plant defense against filamentous pathogens. Front. Plant Sci. 2018, 9, 928. [Google Scholar] [CrossRef]
- Trouvelot, S.; Héloir, M.C.; Poinssot, B.; Gauthier, A.; Paris, F.; Guillier, C.; Combier, M.; Trdá, L.; Daire, X.; Adrian, M. Carbohydrates in plant immunity and plant protection: Roles and potential application as foliar sprays. Front. Plant Sci. 2014, 5, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Tarkowski, Ł.P.; Van de Poel, B.; Höfte, M.; Van den Ende, W. Sweet immunity: Inulin boosts resistance of lettuce (Lactuca sativa) against grey mold (Botrytis cinerea) in an ethylene-dependent manner. Int. J. Mol. Sci. 2019, 20, 1052. [Google Scholar] [CrossRef] [Green Version]
- Mélida, H.; Bacete, L.; Ruprecht, C.; Rebaque, D.; Del Hierro, I.; López, G.; Brunner, F.; Pfrengle, F.; Molina, A. Arabinoxylan-oligosaccharides act as Damage Associated Molecular Patterns in plants regulating disease resistance. Front. Plant Sci. 2020, 11, 1210. [Google Scholar] [CrossRef]
- Iriti, M.; Varoni, E.M. Moving to the field: Plant innate immunity in crop protection. Int. J. Mol. Sci. 2017, 18, 640. [Google Scholar] [CrossRef] [Green Version]
- Ruano-Rosa, D.; Cazorla, F.M.; Bonilla, N.; Martín-Pérez, R.; De Vicente, A.; López-Herrera, C.J. Biological control of avocado white root rot with combined applications of Trichoderma spp. and rhizobacteria. Eur. J. Plant Pathol. 2014, 138, 751–762. [Google Scholar] [CrossRef]
- Djonović, S.; Pozo, M.J.; Dangott, L.J.; Howell, C.R.; Kenerley, C.M. Sm1, a proteinaceous elicitor secreted by the biocontrol fungus Trichoderma virens induces plant defense responses and systemic resistance. Mol. Plant Microbe Interact. 2006, 19, 838–853. [Google Scholar] [CrossRef] [Green Version]
- Li, T.; Fan, P.; Yun, Z.; Jiang, G.; Zhang, Z.; Jiang, Y. β-Aminobutyric acid priming acquisition and defense response of mango fruit to Colletotrichum gloeosporioides infection based on quantitative proteomics. Cells 2019, 8, 1029. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Sheen, J. Dynamic and diverse sugar signaling. Curr. Opin. Plant Biol. 2016, 33, 116–125. [Google Scholar] [CrossRef] [Green Version]
- Lastdrager, J.; Hanson, J.; Smeekens, S. Sugar signals and the control of plant growth and development. J. Exp. Bot. 2014, 65, 799–807. [Google Scholar] [CrossRef]
- Morkunas, I.; Ratajczak, L. The role of sugar signaling in plant defense responses against fungal pathogens. Acta Physiol. Plant. 2014, 36, 1607–1619. [Google Scholar] [CrossRef] [Green Version]
- Janse van Rensburg, H.C.; Van den Ende, W.; Signorelli, S. Autophagy in plants: Both a puppet and a puppet master of sugars. Front. Plant Sci. 2019, 10, 14. [Google Scholar] [CrossRef] [Green Version]
- Bolouri Moghaddam, M.R.; Van den Ende, W. Sweet immunity in the plant circadian regulatory network. J. Exp. Bot. 2013, 64, 1439–1449. [Google Scholar] [CrossRef] [Green Version]
- Morkunas, I.; Marczak, Ł.; Stachowiak, J.; Stobiecki, M. Sucrose-induced lupine defense against Fusarium oxysporum: Sucrose-stimulated accumulation of isoflavonoids as a defense response of lupine to Fusarium oxysporum. Plant Physiol. Biochem. 2005, 43, 363–373. [Google Scholar] [CrossRef]
- Wang, F.; Feng, G.; Chen, K. Burdock fructooligosaccharide induces resistance to tobacco mosaic virus in tobacco seedlings. Physiol. Mol. Plant Pathol. 2009, 74, 34–40. [Google Scholar] [CrossRef]
- He, P.Q.; Tian, L.; Chen, K.S.; Hao, L.H.; Li, G.Y. Induction of volatile organic compounds of Lycopersicon esculentum Mill. and its resistance to Botrytis cinerea Pers. by burdock oligosaccharide. J. Integr. Plant Biol. 2006, 48, 550–557. [Google Scholar] [CrossRef]
- Livingston, D.P.; Hincha, D.K.; Heyer, A.G. Fructan and its relationship to abiotic stress tolerance in plants. Cell. Mol. Life Sci. 2009, 66, 2007–2023. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Livingston, D.P.; Henson, C.A. Apoplastic sugars, fructans, fructan exohydrolase, and invertase in winter oat: Responses to second-phase cold hardening. Plant Physiol. 1998, 116, 403–408. [Google Scholar] [CrossRef] [Green Version]
- Versluys, M.; Tarkowski, Ł.P.; Van den Ende, W. Fructans as DAMPs or MAMPs: Evolutionary prospects, cross-tolerance, and multistress resistance potential. Front. Plant Sci. 2017, 7, 2061. [Google Scholar] [CrossRef] [Green Version]
- Hendry, G.A.F. Evolutionary origins and natural functions of fructans—A climatological, biogeographic and mechanistic appraisal. New Phytol. 1993, 123, 3–14. [Google Scholar] [CrossRef]
- Yáñez, A.; Tapia, G.; Guerra, F.; Del Pozo, A. Stem carbohydrate dynamics and expression of genes involved in fructan accumulation and remobilization during grain growth in wheat (Triticum aestivum L.) genotypes with contrasting tolerance to water stress. PLoS ONE 2017, 12. [Google Scholar] [CrossRef] [Green Version]
- Amiard, V.; Morvan-Bertrand, A.; Billard, J.P.; Huault, C.; Keller, F.; Prud’homme, M.P. Fructans, but not the sucrosyl-galactosides, raffinose and loliose, are affected by drought stress in perennial ryegrass. Plant Physiol. 2003, 132, 2218–2229. [Google Scholar] [CrossRef] [Green Version]
- Gaudet, D.A.; Laroche, A.; Yoshida, M. Low temperature-wheat-fungal interactions: A carbohydrate connection. Physiol. Plant 1999, 106, 437–444. [Google Scholar] [CrossRef]
- Rudd, J.J.; Kanyuka, K.; Hassani-Pak, K.; Derbyshire, M.; Andongabo, A.; Devonshire, J.; Lysenko, A.; Saqi, M.; Desai, N.M.; Powers, S.J.; et al. Transcriptome and metabolite profiling of the infection cycle of Zymoseptoria tritici on wheat reveals a biphasic interaction with plant immunity involving differential pathogen chromosomal contributions and a variation on the hemibiotrophic lifestyle def. Plant Physiol. 2015, 167, 1158–1185. [Google Scholar] [CrossRef] [Green Version]
- Suárez-González, E.M.; López, M.G.; Délano-Frier, J.P.; Gómez-Leyva, J.F. Expression of the 1-SST and 1-FFT genes and consequent fructan accumulation in Agave tequilana and A. inaequidens is differentially induced by diverse (a)biotic-stress related elicitors. J. Plant Physiol. 2014, 171, 359–372. [Google Scholar] [CrossRef] [PubMed]
- Kirtel, O.; Menéndez, C.; Versluys, M.; Van den Ende, W.; Hernández, L.; Toksoy Öner, E. Levansucrase from Halomonas smyrnensis AAD6T: First halophilic GH-J clan enzyme recombinantly expressed, purified, and characterized. Appl. Microbiol. Biotechnol. 2018, 102, 9207–9220. [Google Scholar] [CrossRef] [PubMed]
- Jensen, S.L.; Diemer, M.B.; Lundmark, M.; Larsen, F.H.; Blennow, A.; Mogensen, H.K.; Nielsen, T.H. Levanase from Bacillus subtilis hydrolyses β-2,6 fructosyl bonds in bacterial levans and in grass fructans. Int. J. Biol. Macromol. 2016, 85, 514–521. [Google Scholar] [CrossRef] [PubMed]
- Maleux, K.; Van den Ende, W. Levans in excised leaves of Dactylis glomerata: Effects of light, sugars, temperature and senescence. J. Plant Biol. 2007, 50, 671–680. [Google Scholar] [CrossRef]
- Ferrari, S.; Vairo, D.; Ausubel, F.M.; Cervone, F.; De Lorenzo, G. Tandemly duplicated Arabidopsis genes that encode polygalacturonase-inhibiting proteins are regulated coordinately by different signal transduction pathways in response to fungal infection. Plant Cell 2003, 15, 93–106. [Google Scholar] [CrossRef] [Green Version]
- Albert, M.; Butenko, M.; Aalen, R.; Felix, G.; Wildhagen, M. Chemiluminescence detection of the oxidative burst in plant leaf pieces. Bioprotocolorg 2015, 5. [Google Scholar] [CrossRef]
- Galletti, R.; Denoux, C.; Gambetta, S.; Dewdney, J.; Ausubel, F.M.; De Lorenzo, G.; Ferrari, S. The AtrbohD-mediated oxidative burst elicited by oligogalacturonides in Arabidopsis is dispensable for the activation of defense responses effective against Botrytis cinerea. Plant Physiol. 2008, 148, 1695–1706. [Google Scholar] [CrossRef] [Green Version]
- Cheeseman, J.M. Hydrogen peroxide concentrations in leaves under natural conditions. J. Exp. Bot. 2006, 57, 2435–2444. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.; Zhao, X.; Wu, J.; Hu, M.; Xia, S. The benefits of exogenous NO: Enhancing Arabidopsis to resist Botrytis cinerea. Am. J. Plant Sci. 2011, 02, 511–519. [Google Scholar] [CrossRef] [Green Version]
- Gil-ad, N.L.; Bar-Nun, N.; Noy, T.; Mayer, A.M. Enzymes of Botrytis cinerea capable of breaking down hydrogen peroxide. FEMS Microbiol. Lett. 2000, 190, 121–126. [Google Scholar] [CrossRef] [Green Version]
- Zavaleta-Mancera, H.A.; López-Delgado, H.; Loza-Tavera, H.; Mora-Herrera, M.; Trevilla-García, C.; Vargas-Suárez, M.; Ougham, H. Cytokinin promotes catalase and ascorbate peroxidase activities and preserves the chloroplast integrity during dark-senescence. J. Plant Physiol. 2007, 164, 1572–1582. [Google Scholar] [CrossRef]
- Małolepsza, U.; Urbanek, H. The oxidants and antioxidant enzymes in tomato leaves treated with o-hydroxyethylorutin and infected with Botrytis cinerea. Eur. J. Plant Pathol. 2000, 106, 657–665. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Vergauwen, R.; Van den Ende, W.; Van Laere, A. The role of fructan in flowering of Campanula rapunculoides. J. Exp. Bot. 2000, 51, 1261–1266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zimmerli, L.; Métraux, J.P.; Mauch-Mani, B. β-Aminobutyric acid-induced protection of Arabidopsis against the necrotrophic fungus Botrytis cinerea. Plant Physiol. 2001, 126, 517–523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Po-Wen, C.; Singh, P.; Zimmerli, L. Priming of the Arabidopsis pattern-triggered immunity response upon infection by necrotrophic Pectobacterium carotovorum bacteria. Mol. Plant Pathol. 2013, 14, 58–70. [Google Scholar] [CrossRef] [PubMed]
- Zimmerli, L.; Hou, B.H.; Tsai, C.H.; Jakab, G.; Mauch-Mani, B.; Somerville, S. The xenobiotic β-aminobutyric acid enhances Arabidopsis thermotolerance. Plant J. 2008, 53, 144–156. [Google Scholar] [CrossRef] [Green Version]
- Yoshioka, H.; Bouteau, F.; Kawano, T. Discovery of oxidative burst in the field of plant immunity: Looking back at the early pioneering works and towards the future development. Plant Signal. Behav. 2008, 3, 153–155. [Google Scholar] [CrossRef] [Green Version]
- Baena-González, E.; Rolland, F.; Thevelein, J.M.; Sheen, J. A central integrator of transcription networks in plant stress and energy signalling. Nature 2007, 448, 938–942. [Google Scholar] [CrossRef]
- Baena-González, E. Energy signaling in the regulation of gene expression during stress. Mol. Plant 2010, 3, 300–313. [Google Scholar] [CrossRef] [Green Version]
- Tomé, F.; Nägele, T.; Adamo, M.; Garg, A.; Marco-Llorca, C.; Nukarinen, E.; Pedrotti, L.; Peviani, A.; Simeunovic, A.; Tatkiewicz, A.; et al. The low energy signaling network. Front. Plant Sci. 2014, 5, 353. [Google Scholar] [PubMed] [Green Version]
- Svara, A.; Tarkowski, Ł.P.; Janse van Rensburg, H.C.; Deleye, E.; Vaerten, J.; De Storme, N.; Keulemans, W.; Van den Ende, W. Sweet Immunity: The Effect of Exogenous Fructans on the Susceptibility of Apple (Malus × domestica Borkh.) to Venturia inaequalis. Int. J. Mol. Sci. 2020, 21, 5885. [Google Scholar] [CrossRef]
- Vereyken, I.J.; Chupin, V.; Demel, R.A.; Smeekens, S.C.M.; De Kruijff, B. Fructans insert between the headgroups of phospholipids. Biochim. Biophys. Acta Biomembr. 2001, 1510, 307–320. [Google Scholar] [CrossRef] [Green Version]
- Franco-Robles, E.; López, M.G. Implication of fructans in health: Immunomodulatory and antioxidant mechanisms. Sci. World J. 2015, 2015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vogt, L.M.; Meyer, D.; Pullens, G.; Faas, M.M.; Venema, K.; Ramasamy, U.; Schols, H.A.; de Vos, P. Toll-like receptor 2 activation by β2→1-fructans protects barrier function of t84 human intestinal epithelial cells in a chain length-dependent manner. J. Nutr. 2014, 144, 1002–1008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vogt, L.; Ramasamy, U.; Meyer, D.; Pullens, G.; Venema, K.; Faas, M.M.; Schols, H.A.; de Vos, P. Immune Modulation by Different Types of β2→1-Fructans Is Toll-Like Receptor Dependent. PLoS ONE 2013, 8, e68367. [Google Scholar] [CrossRef] [Green Version]
- De Coninck, B.; Le Roy, K.; Francis, I.; Clerens, S.; Vergauwen, R.; Halliday, A.M.; Smith, S.M.; Van Laere, A.; Van den Ende, W. Arabidopsis AtcwINV3 and 6 are not invertases but are fructan exohydrolases (FEHs) with different substrate specificities. Plant Cell Environ. 2005, 28, 432–443. [Google Scholar] [CrossRef]
- Camejo, D.; Guzmán-Cedeño, Á.; Moreno, A. Reactive oxygen species, essential molecules, during plant-pathogen interactions. Plant Physiol. Biochem. 2016, 103, 10–23. [Google Scholar] [CrossRef]
- Kimura, S.; Waszczak, C.; Hunter, K.; Wrzaczek, M. Bound by fate: The role of reactive oxygen species in receptor-like kinase signaling. Plant Cell 2017, 29, 638–654. [Google Scholar] [CrossRef] [Green Version]
- Chaouch, S.; Queval, G.; Noctor, G. AtRbohF is a crucial modulator of defence-associated metabolism and a key actor in the interplay between intracellular oxidative stress and pathogenesis responses in Arabidopsis. Plant J. 2012, 69, 613–627. [Google Scholar] [CrossRef]
- Kadota, Y.; Sklenar, J.; Derbyshire, P.; Stransfeld, L.; Asai, S.; Ntoukakis, V.; Jones, J.D.; Shirasu, K.; Menke, F.; Jones, A.; et al. Direct regulation of the NADPH oxidase RBOHD by the PRR-associated kinase BIK1 during plant immunity. Mol. Cell 2014, 54, 43–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qi, J.; Wang, J.; Gong, Z.; Zhou, J.M. Apoplastic ROS signaling in plant immunity. Curr. Opin. Plant Biol. 2017, 38, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Kuźniak, E.; Skłodowska, M. Ascorbate, glutathione and related enzymes in chloroplasts of tomato leaves infected by Botrytis cinerea. Plant Sci. 2001, 160, 723–731. [Google Scholar] [CrossRef]
- Kuźniak, E.; Sklodowska, M. The effect of Botrytis cinerea infection on ascorbate-glutathione cycle in tomato leaves. Plant Sci. 1999, 148, 69–76. [Google Scholar] [CrossRef]
- Nowogórska, A.; Patykowski, J. Selected reactive oxygen species and antioxidant enzymes in common bean after Pseudomonas syringae pv. phaseolicola and Botrytis cinerea infection. Acta Physiol. Plant. 2015, 37. [Google Scholar] [CrossRef] [Green Version]
- Kuźniak, E.; Sklłodowska, M. The effect of Botrytis cinerea infection on the antioxidant profile of mitochondria from tomato leaves. J. Exp. Bot. 2004, 55, 605–612. [Google Scholar] [CrossRef]
- Simon, U.K.; Polanschütz, L.M.; Koffler, B.E.; Zechmann, B. High resolution imaging of temporal and spatial changes of subcellular ascorbate, glutathione and H2O2 distribution during Botrytis cinerea infection in Arabidopsis. PLoS ONE 2013, 8, e65811. [Google Scholar] [CrossRef] [Green Version]
- Couée, I.; Sulmon, C.; Gouesbet, G.; El Amrani, A. Involvement of soluble sugars in reactive oxygen species balance and responses to oxidative stress in plants. J. Exp. Bot. 2006, 57, 449–459. [Google Scholar] [CrossRef]
- Keunen, E.; Peshev, D.; Vangronsveld, J.; Van den Ende, W.; Cuypers, A. Plant sugars are crucial players in the oxidative challenge during abiotic stress: Extending the traditional concept. Plant Cell Environ. 2013, 36, 1242–1255. [Google Scholar] [CrossRef]
- Li, Y.; Van den Ende, W.; Rolland, F. Sucrose induction of anthocyanin biosynthesis is mediated by della. Mol. Plant 2014, 7, 570–572. [Google Scholar] [CrossRef] [Green Version]
- Sade, D.; Brotman, Y.; Eybishtz, A.; Cuadros-Inostroza, Á.; Fernie, A.R.; Willmitzer, L.; Czosnek, H. Involvement of the hexose transporter gene LeHT1 and of sugars in resistance of tomato to tomato yellow leaf curl virus. Mol. Plant 2013, 6, 1707–1710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, F.; Zhu, L.; Wang, X.; Cheng, J.; Cui, B.; Liu, J.; Tan, F.; Fu, M. Sucrose transportation control mediates the fresh-keeping effects of burdock fructooligosaccharide in ‘Crimson Seedless’ grapes. Food Chem. 2020, 332. [Google Scholar] [CrossRef] [PubMed]
- Chong, J.; Piron, M.C.; Meyer, S.; Merdinoglu, D.; Bertsch, C.; Mestre, P. The SWEET family of sugar transporters in grapevine: VvSWEET4 is involved in the interaction with Botrytis cinerea. J. Exp. Bot. 2014, 65, 6589–6601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shtienberg, D.; Elad, Y.; Niv, A.; Nitzani, Y.; Kirshner, B. Significance of leaf infection by Botrytis cinerea in stem rotting of tomatoes grown in non-heated greenhouses. Eur. J. Plant Pathol. 1998, 104, 753–763. [Google Scholar] [CrossRef]
- Michaeli, S.; Fromm, H. Closing the loop on the GABA shunt in plants: Are GABA metabolism and signaling entwined? Front. Plant Sci. 2015, 6, 419. [Google Scholar] [CrossRef] [Green Version]
- Muckenschnabel, I.; Goodman, B.A.; Williamson, B.; Lyon, G.D.; Deighton, N. Infection of leaves of Arabidopsis thaliana by Botrytis cinerea: Changes in ascorbic acid, free radicals and lipid peroxidation products. J. Exp. Bot. 2002, 53, 207–214. [Google Scholar] [CrossRef] [Green Version]
- Angelos, E.; Brandizzi, F. NADPH oxidase activity is required for ER stress survival in plants. Plant J. 2018, 96, 1106–1120. [Google Scholar] [CrossRef] [Green Version]
- Pu, Y.; Bassham, D.C. Links between ER stress and autophagy in plants. Plant Signal. Behav. 2013, 8, e24297. [Google Scholar] [CrossRef] [Green Version]
- Srivastava, R.; Li, Z.; Russo, G.; Tang, J.; Bi, R.; Muppirala, U.; Chudalayandi, S.; Severin, A.; He, M.; Vaitkevicius, S.I.; et al. Response to persistent er stress in plants: A multiphasic process that transitions cells from prosurvival activities to cell death. Plant Cell 2018, 30, 1220–1242. [Google Scholar] [CrossRef] [Green Version]







| Control | Inulin | p95 | BFOs | Levan | LOS | Dactylis | |
|---|---|---|---|---|---|---|---|
| % Germination | 89.11 | 89.32 | 92.29 | 89.59 | 93.51 | 92.89 | 81.70 |
| SD | 2.82 | 1.65 | 0.99 | 1.44 | 4.97 | 1.28 | 3.79 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Janse van Rensburg, H.C.; Takács, Z.; Freynschlag, F.; Toksoy Öner, E.; Jonak, C.; Van den Ende, W. Fructans Prime ROS Dynamics and Botrytis cinerea Resistance in Arabidopsis. Antioxidants 2020, 9, 805. https://doi.org/10.3390/antiox9090805
Janse van Rensburg HC, Takács Z, Freynschlag F, Toksoy Öner E, Jonak C, Van den Ende W. Fructans Prime ROS Dynamics and Botrytis cinerea Resistance in Arabidopsis. Antioxidants. 2020; 9(9):805. https://doi.org/10.3390/antiox9090805
Chicago/Turabian StyleJanse van Rensburg, Henry Christopher, Zoltan Takács, Florentina Freynschlag, Ebru Toksoy Öner, Claudia Jonak, and Wim Van den Ende. 2020. "Fructans Prime ROS Dynamics and Botrytis cinerea Resistance in Arabidopsis" Antioxidants 9, no. 9: 805. https://doi.org/10.3390/antiox9090805