Diabetes Upregulates Oxidative Stress and Downregulates Cardiac Protection to Exacerbate Myocardial Ischemia/Reperfusion Injury in Rats
Abstract
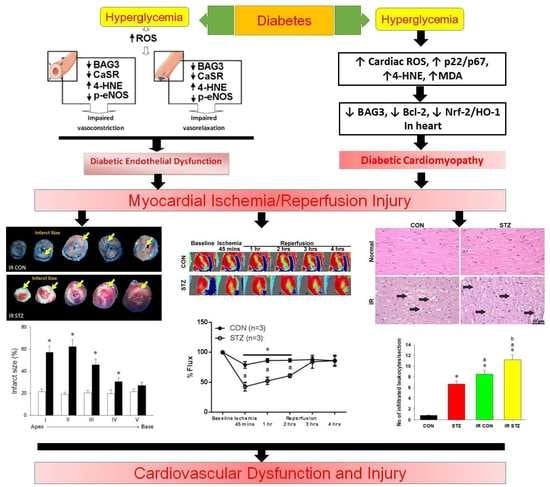
:1. Introduction
2. Methods and Materials
2.1. Animals
2.2. Grouping
2.3. Type I Diabetes Induction
2.4. Metabolic Cage Analysis
2.5. Evaluation of Cardiac Hemodynamic Parameters
2.6. Induction of Myocardial Ischemia/Reperfusion
2.7. Heart Microcirculation Determination
2.8. In vivo Real-Time Chemiluminescence Recording of Heart Superoxide Anion Activity
2.9. Mesenteric Arterial Wire Myography
2.10. Western Blot
2.11. Infarct Size Calculation
2.12. In situ Demonstration of Fibrosis, Oxidative Stress and Apoptosis Formation
2.13. MDA Assay
2.14. Statistical Analysis
3. Results
3.1. STZ Induced Type I Diabetes Led to Decreased Body Weight and Hyperglycemia
3.2. STZ Induced Type I Diabetes Evoked Polydipsia, Polyuria and Polyphagia
3.3. Diabetes Affects Mesenteric Arterial Proteins Expression and Responses to Vasoactive Agents
3.4. Diabetes Exacerbated Myocardial IR Induced Left Ventricular Dysfunction
3.5. Diabetes Exacerbated Oxidative Stress and Depressed Antioxidant Defense in the Heart
3.6. Diabetes Further Exacerbated Myocardial IR Evoked Oxidative Stress, Inflammation and Injury
3.7. Diabetes Exacerbated Myocardial IR Depressed Cardiac BAG3 Expression
3.8. Diabetes Exacerbated Myocardial IR Depressed Cardiac Bcl-2 Expression
3.9. Diabetes Exacerbated Myocardial IR Enhanced Apoptosis Formation
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| 4-HNE | 4-hydroxynonenal |
| ACh | acetylcholine |
| BAG3 | Bcl-2-associated athanogene 3 |
| Bcl-2 | B-cell lymphoma 2 |
| +dp/dt | peak rate of pressure increase |
| −dp/dt | peak rate of pressure decrease |
| Drp1 | dynamin-related protein 1 |
| eNOS | endothelial nitric oxide synthase |
| GSK-3β | glycogen synthase kinase-3 beta |
| GULT | glucose transporter |
| H&E stain | hematoxylin and eosin stain |
| IR | ischemia reperfusion |
| JAK2 | Janus kinase 2 |
| LVEDP | left ventricular end-diastolic pressure |
| MDA | malondialdehyde |
| NE | norepinephrine |
| PARP | poly (ADP-ribose) polymerase |
| PI3K | phosphoinositide 3-kinase |
| PTEN | phosphatase and tensin homologue deleted on chromosome 10 |
| ROS | reactive oxygen species |
| Sirt1 | sirtuin1 |
| STAT3 | signal transducer and activator of transcription 3 |
| STZ | streptozotocin |
| TUNEL | terminal deoxynucleotidyl transferase dUTP nick end labeling |
References
- Ahmad, F.S.; Ning, H.; Rich, J.D.; Yancy, C.W.; Donald MLloyd-Jones, D.M.; Wilkins, J.T. Hypertension, Obesity, Diabetes, and Heart Failure-Free Survival: The Cardiovascular Disease Lifetime Risk Pooling Project. JACC Heart Fail. 2016, 4, 911–919. [Google Scholar] [CrossRef] [PubMed]
- Mozaffarian, D.; Benjamin, E.J.; Go, A.S.; Arnett, D.K.; Blaha, M.J.; Cushman, M.; Das, S.R.; de Ferranti, S.; Després, J.P.; Fullerton, H.J.; et al. Executive Summary: Heart Disease and Stroke Statistics—2016 Update: A Report From the American Heart Association. Circulation 2016, 133, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Haffner, S.M.; Lehto, S.; Rönnemaa, T.; Pyörälä, K.; Laakso, M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N. Engl. J. Med. 1998, 339, 229–234. [Google Scholar] [CrossRef]
- Quertermous, T.; Ingelsson, E. Coronary artery disease and its risk factors: Leveraging shared genetics to discover novel biology. Circ. Res. 2016, 118, 14–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahalakshmi, A.; Kurian, G.A. Evaluating the impact of diabetes and diabetic cardiomyopathy rat heart on the outcome of ischemia-reperfusion associated oxidative stress. Free Radic. Biol. Med. 2018, 118, 35–43. [Google Scholar] [CrossRef]
- Lei, S.; Su, W.; Xia, Z.Y.; Wang, Y.; Zhou, L.; Qiao, S.; Zhao, B.; Xia, Z.; Irwin, M.G. Standards of Medical Care in Diabetes. Diabetes Care 2019, 42, S81. [Google Scholar]
- Selvin, E.; Marinopoulos, S.; Berkenblit, G.; Rami, T.; Brancati, F.L.; Powe, N.R.; Golden, S.H. Meta-analysis: Glycosylated hemoglobin and cardiovascular disease in diabetes mellitus. Ann. Intern. Med. 2004, 141, 421–431. [Google Scholar] [CrossRef]
- Gerstein, H.C.; Islam, S.; Anand, S.; Almahmeed, W.; Damasceno, A.; Dans, A.; Lang, C.C.; Luna, M.A.; McQueen, M.; Rangarajan, S.; et al. Dysglycaemia and the risk of acute myocardial infarction in multiple ethnic groups: An analysis of 15,780 patients from the INTERHEART study. Diabetologia 2010, 53, 2509–2517. [Google Scholar] [CrossRef] [Green Version]
- Gerstein, H.C.; Pogue, J.; Mann, J.F.; Lonn, E.; Dagenais, G.R.; McQueen, M.; Yusuf, S. HOPE investigators. The relationship between dysglycaemia and cardiovascular and renal risk in diabetic and non-diabetic participants in the HOPE study: A prospective epidemiological analysis. Diabetologia 2005, 48, 1749–1755. [Google Scholar] [CrossRef] [Green Version]
- Sena, C.M.; Pereira, A.M.; Seiça, R. Endothelial dysfunction—A major mediator of diabetic vascular disease. Biochim. Biophys. Acta Mol. Basis Dis. 2013, 1832, 2216–2231. [Google Scholar] [CrossRef] [Green Version]
- Brownlee, M. Biochemistry and molecular cell biology of diabetic complications. Nature 2001, 414, 813. [Google Scholar] [CrossRef] [PubMed]
- Su, F.; Myers, V.D.; Knezevic, T.; Wang, J.; Gao, E.; Madesh, M.; Tahrir, F.G.; Gupta, M.K.; Gordon, J.; Rabinowitz, J.; et al. Bcl-2-associated athanogene 3 protects the heart from ischemia/reperfusion injury. JCI Insight 2016, 1, e90931. [Google Scholar] [CrossRef]
- Li, H.; Liu, Z.; Wang, J.; Wong, G.T.; Cheung, C.W.; Zhang, L.; Chen, C.; Xia, Z.; Irwin, M.G. Susceptibility to myocardial ischemia reperfusion injury at early stage of type 1 diabetes in rats. Cardiovasc. Diabetol. 2013, 12, 133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, T.; Mao, X.; Li, H.; Qiao, S.; Xu, A.; Wang, J.; Lei, S.; Liu, Z.; Ng, K.F.; Wong, G.T.; et al. N-Acetylcysteine and allopurinol up-regulated the Jak/STAT3 and PI3K/Akt pathways via adiponectin and attenuated myocardial postischemic injury in diabetes. Free Radic. Biol. Med. 2013, 63, 291–303. [Google Scholar] [CrossRef] [PubMed]
- Tao, A.; Xu, X.; Kvietys, P.; Kao, R.; Martin, C.; Rui, T. Experimental diabetes mellitus exacerbates ischemia/reperfusion-induced myocardial injury by promoting mitochondrial fission: Role of down-regulation of myocardial Sirt1 and subsequent Akt/Drp1 interaction. Int. J. Biochem. Cell Biol. 2018, 105, 94–103. [Google Scholar] [CrossRef]
- Li, H.; Shi, Y.; Wang, X.; Li, P.; Zhang, S.; Wu, T.; Yan, Y.; Zhan, Y.; Ren, Y.; Rong, X.; et al. Piceatannol alleviates inflammation and oxidative stress via modulation of the Nrf2/HO-1 and NF-κB pathways in diabetic cardiomyopathy. Chem. Biol. Interact. 2019, 310, 108754. [Google Scholar] [CrossRef]
- Knezevic, T.; Myers, V.D.; Gordon, J.; Tilley, D.G.; Sharp, T.E., 3rd; Wang, J.; Khalili, K.; Cheung, J.Y.; Feldman, A.M.; Knezevic, T. BAG3: A new player in the heart failure paradigm. Heart Fail. Rev. 2015, 20, 423–434. [Google Scholar] [CrossRef] [Green Version]
- Minoia, M.; Boncoraglio, A.; Vinet, J.; Morelli, F.; Brunsting, J.F.; Poletti, A.; Carra, S. BAG3 induces the sequestration of proteasomal clients into cytoplasmic puncta: Implications for a proteasome-to-autophagy switch. Autophagy 2014, 10, 1603–1621. [Google Scholar] [CrossRef] [Green Version]
- Schänzer, A.; Rupp, S.; Gräf, S.; Zengeler, D.; Jux, C.; Akintürk, H.; Hahn, A. Dysregulated autophagy in restrictive cardiomyopathy due to Pro209Leu mutation in BAG3. Mol. Genet. Metab. 2018, 123, 388–399. [Google Scholar] [CrossRef]
- Homma, S.; Iwasaki, M.; Shelton, G.D.; Engvall, E.; Reed, J.C.; Takayama, S. BAG3 deficiency results in fulminant myopathy and early lethality. Am. J. Pathol. 2006, 169, 761–773. [Google Scholar] [CrossRef] [Green Version]
- Tahrir, F.G.; Knezevic, T.; Gupta, M.K.; Gordon, J.; Cheung, J.Y.; Feldman, A.M.; Khalili, K. Evidence for the role of BAG3 in mitochondrial quality control in cardiomyocytes. J. Cell. Physiol. 2017, 232, 797–805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carrizzo, A.; Damato, A.; Ambrosio, M.; Falco, A.; Rosati, A.; Capunzo, M.; Madonna, M.; Turco, M.C.; Januzzi, J.L.; Laurenzi, V.D.; et al. The prosurvival protein BAG3: A new participant in vascular homeostasis. Cell Death Dis. 2016, 7, e2431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loot, A.E.; Pierson, I.; Syzonenko, T.; Elgheznawy, A.; Randriamboavonjy, V.; Zivković, A.; Stark, H.; Fleming, I. Ca2+-sensing receptor cleavage by calpain partially accounts for altered vascular reactivity in mice fed a high-fat diet. J. Cardiovasc. Pharmacol. 2013, 61, 528–535. [Google Scholar] [CrossRef]
- Bellezza, I.; Riuzzi, F.; Chiappalupi, S.; Arcuri, C.; Giambanco, I.; Sorci, G.; Donato, R. Reductive stress in striated muscle cells. Cell. Mol. Life Sci. 2020. Accepted. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Chang, J.J.; Chien, C.T.; Yang, M.C.; Chien, H.F. Antioxidant sol-gel improves cutaneous wound healing in streptozotocin-induced diabetic rats. Exp. Diabetes Res. 2012, 2012, 504693. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.H.; Chiang, C.Y.; Chang, T.C.; Chien, C.T. Multiple Progressive thermopreconditioning improves cardiac ischemia/reperfusion induced left ventricular contractile dysfunction and structural abnormality in rat. Transplantation 2020. Accepted. [Google Scholar] [CrossRef]
- Chien, C.Y.; Chien, C.T.; Wang, S.S. Progressive thermopreconditioning attenuates rat cardiac ischemia/reperfusion injury by mitochondria-mediated antioxidant and antiapoptotic mechanisms. J. Thorac. Cardiovasc. Surg. 2014, 148, 705–713. [Google Scholar] [CrossRef] [Green Version]
- Chiang, C.Y.; Chien, C.Y.; Qiou, W.Y.; Chang, C.; Yu, I.S.; Chang, P.Y.; Chien, C.T. Genetic depletion of thromboxane A2/thromboxane-prostanoid receptor signalling prevents microvascular dysfunction in ischaemia/reperfusion injury. Thromb. Haemost. 2018, 118, 1982–1996. [Google Scholar] [CrossRef]
- Przyklenk, K.; Maynard, M.; Greiner, D.L.; Whittaker, P. Cardioprotection with postconditioning: Loss of efficacy in murine models of type-2 and type-1 diabetes. Antioxid. Redox Signal. 2011, 14, 781–790. [Google Scholar] [CrossRef] [Green Version]
- Tsakadze, N.L.; Srivastava, S.; Awe, S.O.; Adeagbo, A.S.; Bhatnagar, A.; D’Souza, S.E. Acrolein-induced vasomotor responses of rat aorta. Am. J. Physiol. Heart Circ. Physiol. 2003, 285, H727–H734. [Google Scholar] [CrossRef] [Green Version]
- Arshad, M.; Vijay, V.; Floyd, B.C.; Marks, B.; Sarabu, M.R.; Wolin, M.S.; Gupte, S.A. Thromboxane receptor stimulation suppresses guanylate cyclase-mediated relaxation of radial arteries. Ann. Thorac. Surg. 2006, 81, 2147–2154. [Google Scholar] [CrossRef]
- Minarchick, V.C.; Stapleton, P.A.; Porter, D.W.; Wolfarth, M.G.; Çiftyürek, E.; Barger, M.; Sabolsky, E.M.; Nurkiewicz, T.R. Pulmonary cerium dioxide nanoparticle exposure differentially impairs coronary and mesenteric arteriolar reactivity. Cardiovasc. Toxicol. 2013, 13, 323–337. [Google Scholar] [CrossRef] [Green Version]
- Zhang, R.; Thor, D.; Han, X.; Anderson, L.; Rahimian, R. Sex differences in mesenteric endothelial function of streptozotocin-induced diabetic rats: A shift in the relative importance of EDRFs. Am. J. Physiol. Heart Circ. Physiol. 2012, 303, H1183–H1198. [Google Scholar] [CrossRef] [Green Version]
- Park, S.H.; Bahk, J.H.; Oh, A.Y.; Gil, N.S.; Huh, J.; Lee, J.H. Gender difference and change of α1-adrenoceptors in the distal mesenteric arteries of streptozotocin-induced diabetic rats. Korean J. Anesthesiol. 2011, 61, 419. [Google Scholar] [CrossRef] [PubMed]
- Zaugg, C.E.; Hornstein, P.S.; Zhu, P.; Simper, D.; Lüscher, T.F.; Allegrini, P.R.; Buser, P.T. Endothelin-1-induced release of thromboxane A2 increases the vasoconstrictor effect of endothelin-1 in postischemic reperfused rat hearts. Circulation 1996, 94, 742–747. [Google Scholar] [CrossRef] [PubMed]
- Ansley, D.M.; Wang, B. Oxidative stress and myocardial injury in the diabetic heart. J. Pathol. 2013, 229, 232–241. [Google Scholar] [CrossRef] [Green Version]
- Xia, Z.; Vanhoutte, P.M. Nitric oxide and protection against cardiac ischemia. Curr. Pharm. Des. 2011, 17, 1774–1782. [Google Scholar] [CrossRef]
- Galiñanes, M.; Fowler, A.G. Role of clinical pathologies in myocardial injury following ischaemia and reperfusion. Cardiovasc. Res. 2004, 61, 512–521. [Google Scholar] [CrossRef]
- Xue, R.; Lei, S.; Xia, Z.; Wu, Y.; Meng, Q.; Zhan, L.; Su, W.; Liu, H.; Xu, X.; Liu, Z.; et al. Selective inhibition of PTEN preserves ischaemic post-conditioning cardioprotection in STZ-induced Type 1 diabetic rats: Role of the PI3K/Akt and JAK2/STAT3 pathways. Clin. Sci. 2016, 130, 377–392. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Wu, F.; Luo, T.; Zhang, Y. CCAAT/enhancer binding protein homologous protein knockdown alleviates hypoxia-induced myocardial injury in rat cardiomyocytes exposed to high glucose. Exp. Ther. Med. 2018, 15, 4213–4222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pacher, P.; Szabo, C. Role of poly(ADP-ribose) polymerase 1 (PARP-1) in cardiovascular diseases: The therapeutic potential of PARP inhibitors. Cardiovasc. Drug Rev. 2007, 25, 235–260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, N.; Yang, Z.; Xiang, S.Z.; Jin, Y.G.; Wei, W.Y.; Bian, Z.Y.; Deng, W.; Tang, Q.Z. Nobiletin attenuates cardiac dysfunction, oxidative stress, and inflammatory in streptozotocin: Induced diabetic cardiomyopathy. Mol. Cell. Biochem. 2016, 417, 87–96. [Google Scholar] [CrossRef] [PubMed]








© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chien, C.-Y.; Wen, T.-J.; Cheng, Y.-H.; Tsai, Y.-T.; Chiang, C.-Y.; Chien, C.-T. Diabetes Upregulates Oxidative Stress and Downregulates Cardiac Protection to Exacerbate Myocardial Ischemia/Reperfusion Injury in Rats. Antioxidants 2020, 9, 679. https://doi.org/10.3390/antiox9080679
Chien C-Y, Wen T-J, Cheng Y-H, Tsai Y-T, Chiang C-Y, Chien C-T. Diabetes Upregulates Oxidative Stress and Downregulates Cardiac Protection to Exacerbate Myocardial Ischemia/Reperfusion Injury in Rats. Antioxidants. 2020; 9(8):679. https://doi.org/10.3390/antiox9080679
Chicago/Turabian StyleChien, Chen-Yen, Ting-Jui Wen, Yu-Hsiuan Cheng, Yi-Ting Tsai, Chih-Yao Chiang, and Chiang-Ting Chien. 2020. "Diabetes Upregulates Oxidative Stress and Downregulates Cardiac Protection to Exacerbate Myocardial Ischemia/Reperfusion Injury in Rats" Antioxidants 9, no. 8: 679. https://doi.org/10.3390/antiox9080679