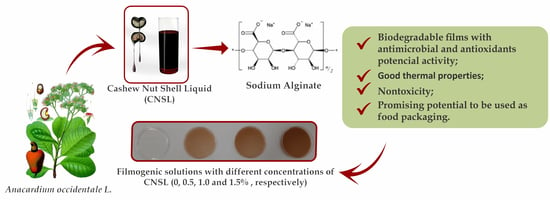
Elaboration and Characterization of Bioactive Films Obtained from the Incorporation of Cashew Nut Shell Liquid into a Matrix of Sodium Alginate
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material
2.2. Elaboration of the Biopolymer Films
2.3. Characterization of Films
2.3.1. Thermogravimetric Analysis (TGA) and Differential Scanning Calorimetry (DSC)
2.3.2. Color and Opacity
2.3.3. Morphology of the Films
2.3.4. Thickness
2.3.5. Water Vapor Permeability
2.3.6. Mechanical Properties
2.3.7. Antimicrobial Activity of the CNSL
2.3.8. Antibacterial Activity of the Films—Agar Well Diffusion Method
2.3.9. Determination of Antioxidant Activity of CNSL
2.3.10. Determination of the Antioxidant Activity of the Films
2.3.11. Toxicity Test
2.4. Statistical Analysis
3. Results
3.1. Thermogravimetric Analysis (TGA) and Differential Scanning Calorimetry (DSC)
3.2. Morphology
3.3. Color Parameters, Mechanical Proprieties, Water Vapor Permeability, and Thickness
3.4. Antimicrobial Activity
3.5. Antioxidant Activity
3.6. Toxicity Test
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Popović, S.Z.; Lazić, V.L.; Hromiš, N.M.; Šuput, D.Z.; Bulut, S.N. Biopolymer Packaging Materials for Food Shelf-Life Prolongation. Biopolym. Food Des. 2015, 8, 223–277. [Google Scholar] [CrossRef]
- Otoni, C.G.; Avena-Bustillos, R.J.; Azeredo, H.M.C.; Lorevice, M.V.; Moura, M.R.; Mattoso, L.H.C.; McHugh, T.H. Recent Advances on Edible Films Based on Fruits and Vegetables-A Review. Compr. Rev. Food Sci. Food Saf. 2017, 16, 1151–1169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baschetti, M.G.; Minelli, M. Test methods for the characterization of gas and vapor permeability in polymers for food packaging application: A review. Polym. Test. 2020, 89, 106606. [Google Scholar] [CrossRef]
- Muller, J.; González-Martínez, C.; Chiralt, A. Combination of Poly(lactic) Acid and Starch for Biodegradable Food Packaging. Materials 2017, 10, 952. [Google Scholar] [CrossRef] [PubMed]
- Cox, K.D.; Covernton, G.A.; Davies, H.L.; Dower, J.F.; Juanes, F.; Dudas, S.E. Human Consumption of Microplastics. Environ. Sci. Technol. 2019, 53, 7068–7074. [Google Scholar] [CrossRef] [Green Version]
- Khodaei, D.; Álvarez, C.; Mullen, A.M. Biodegradable Packaging Materials from Animal Processing Co-Products and Wastes: An Overview. Polymers 2021, 13, 2561. [Google Scholar] [CrossRef] [PubMed]
- Menossi, M.; Cisneros, M.; Alvarez, V.A.; Casalongué, C. Current and emerging biodegradable mulch films based on polysaccharide bio-composites. A review. Agron. Sustain. Dev. 2021, 41, 53. [Google Scholar] [CrossRef]
- Souza, M.P.; Vaz, A.F.M.; Silva, H.D.; Cerqueira, M.A.; Vicente, A.A.; Carneiro-da-Cunha, M.G. Development and Characterization of an Active Chitosan-Based Film Containing Quercetin. Food Bioprocess Technol. 2015, 8, 2183–2191. [Google Scholar] [CrossRef] [Green Version]
- Lim, C.; Yusoff, S.; Ng, C.G.; Lim, P.E.; Ching, Y.C. Bioplastic made from seaweed polysaccharides with green production methods. J. Environ. Chem. Eng. 2021, 9, 105895. [Google Scholar] [CrossRef]
- Mohamed, S.A.A.; El-Sakhawy, M.; El-Sakhawy, M.A.M. Polysaccharides, protein and lipid- Based Natural Edible Films in Food Packaging: A Review. Carbohydr. Polym. 2020, 116178. [Google Scholar] [CrossRef]
- Umaraw, P.; Munekata, P.E.S.; Verma, A.K.; Barba, F.J.; Singh, V.P.; Kumar, P.; Lorenzo, J.M. Edible films/coating with tailored properties for active packaging of meat, fish and derived products. Trends Food Sci. Technol. 2020, 98, 10–24. [Google Scholar] [CrossRef]
- Rodrigues, F.M.G.; Souza, A.G.; Santos, I.M.G.; Bicudo, T.C.; Silva, M.C.D.; Sinfrônio, F.S.M.; Vasconselos, A.F.F. Antioxidative properties of hydrogenated cardanol for cotton biodiesel by PDSC and UV/VIS. J. Therm. Anal. Calorim. 2009, 97, 605–609. [Google Scholar] [CrossRef]
- Rodrigues, F.H.A.; Feitosa, J.P.A.; Ricardo, N.M.P.S.; de França, F.C.F.; Carioca, J.O.B. Antioxidant activity of cashew nut shell liquid (CNSL) derivatives on the thermal oxidation of synthetic cis-1,4-polyisoprene. J. Braz. Chem. Soc. 2006, 17, 265–271. [Google Scholar] [CrossRef]
- Citó, A.M.D.G.L.; da Silva, J.; Saffi, J.; Richter, M.F.; Ferraz, A.D.B.F. Antioxidant properties and chemical composition of technical Cashew Nut Shell Liquid (tCNSL). Food Chem. 2011, 126, 1044–1048. [Google Scholar] [CrossRef]
- Topuz, F.; Uyar, T. Antioxidant, Antibacterial and Antifungal Electrospun Nanofibers for Food Packaging Applications. Food Res. Int. J. 2019, 130, 108927. [Google Scholar] [CrossRef]
- Oussalah, M.; Caillet, S.; Salmiéri, S.; Saucier, L.; Lacroix, M. Antimicrobial Effects of Alginate-Based Film Containing Essential Oils for the Preservation of Whole Beef Muscle. J. Food Prot. 2006, 69, 2364–2369. [Google Scholar] [CrossRef] [PubMed]
- Boccard, R.; Buchter, L.; Casteels, E.; Cosentino, E.; Dransfield, E.; Hood, D.E.; Joseph, R.L.; McDougall, D.B.; Touraille, C. Procedures for measuring meat quality characteristics in beef production experiments. Report of a working group in the Commission of the European Communities (CEC) Beef Production Research Programme. Livest. Prod. Sci. 1981, 8, 385–397. [Google Scholar] [CrossRef]
- ASTM-American Society for Testing and Material. ASTM D882- 12: Standard Test Method for Tensile Properties of Thin Plastic Sheeting; ASTM: West Conshohocken, PA, USA, 2016. [Google Scholar]
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing, 28th ed.; Clinical Laboratory Standards Institute: Wayne, PA, USA, 2018. [Google Scholar]
- Blois, M.S. Antioxidant Determinations by the Use of a Stable Free Radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Prieto, P.; Pineda, M.; Aguilar, M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: Specific application to the determination of vitamin E. Anal. Biochem. 1999, 269, 337–341. [Google Scholar] [CrossRef]
- Meyer, B.N.; Ferrigni, N.R.; Putnam, J.E.; Jacobsen, L.B.; Nichols, D.E.; McLaughin, J.L. Brine Shrimp: A Convenient General Bioassay for Active Plant Constituents. J. Med. Plant Res. 1982, 45, 31–34. [Google Scholar] [CrossRef]
- Daza, L.D.; Homez-Jara, A.; Solanilla, J.F.; Váquiro, H.A. Effects of temperature, starch concentration, and plasticizer concentration on the physical properties of ulluco (Ullucus tuberosus Caldas)-based edible films. Int. J. Biol. Macromol. 2018, 120 Pt B, 1834–1845. [Google Scholar] [CrossRef]
- Celso, F.; Mauler, R.S.; Gomes, A.S. Thermal Properties of Speek-based Polymeric Films Containing Benzimidazole Derivatives and Fosfotungstic Acid. Polímeros Cien. Tecnol. 2008, 18, 178–186. [Google Scholar] [CrossRef] [Green Version]
- Mazzetto, S.E.; Lomonaco, D.; Mele, G. Cashew nut oil: Opportunities and challenges in the context of sustainable industrial development. Quím. Nova 2009, 32, 732–741. [Google Scholar] [CrossRef] [Green Version]
- Chuayjuljit, S.; Rattanametangkool, P.; Potiyaraj, P. Preparation of cardanol–formaldehyde resins from cashew nut shell liquid for the reinforcement of natural rubber. J. Appl. Polym. Sci. 2007, 104, 1997–2002. [Google Scholar] [CrossRef]
- Prasad, V.S.; Pillai, C.K.S. Polwtzer Science-Recent Advances, 1st ed.; Bhardwaj, S., Ed.; Allied Publ. Ltd.: New Delhi, India, 1994; Volume 2. [Google Scholar]
- Menon, A.R.R.; Pillai, C.K.S.; Nando, G.B. Thermal degradation characteristics of natural rubber vulcanizates modified with phosphorylated cashew nut shell liquid. Polym. Degrad. Stab. 1996, 52, 265–271. [Google Scholar] [CrossRef]
- Yang, M.; Wang, L.; Xia, Y. Ammonium persulphate induced synthesis of polymethyl methacrylate grafted sodium alginate composite films with high strength for food packaging. Int. J. Biol. Macromol. 2019, 124, 1238–1245. [Google Scholar] [CrossRef]
- Nawab, A.; Alam, F.; Haq, M.A.; Lutfi, Z.; Hasnain, A. Mango kernel starch-gum composite films: Physical, mechanical and barrier properties. Int. J. Biol. Macromol. 2017, 98, 869–876. [Google Scholar] [CrossRef] [PubMed]
- Wasserman, D.; Dawson, C.R. Cashew nut shell liquid. III. The cardol component of Indian cashew nut shell liquid with reference to the liquid s vesicant activity. J. Am. Chem. Soc. 1948, 70, 3675–3679. [Google Scholar] [CrossRef]
- Homez-JFara, A.; Daza, L.D.; Aguirre, D.M.; Muñoz, J.A.; Solanilla, J.F.; Váquiro, H.A. Characterization of chitosan edible films obtained with various polymer concentrations and drying temperatures. Int. J. Biol. Macromol. 2018, 113, 1233–1240. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, A.P.S.; Costa, J.B.; Soares, D.S.B.; de Moura, C.J.; de Souza, A.R.M. Application of biodegradable films produced from irradiated whey protein concentrate. Pesq. Agropec. Trop. 2015, 45, 192–199. [Google Scholar] [CrossRef] [Green Version]
- Do Evangelho, J.A.; da Silva Dannenberg, G.; Biduski, B.; el Halal, S.L.M.; Kringel, D.H.; Gularte, M.A.; da Rosa Zavareze, E. Antibacterial activity, optical, mechanical, and barrier properties of corn starch films containing orange essential oil. Carbohydr. Polym. 2019, 222, 114981. [Google Scholar] [CrossRef] [PubMed]
- Ghelejlu, S.B.; Esmaiili, M.H.; Almasi, H. Characterization of chitosan–nanoclay bionanocomposite active films containing milk thistle extract. Int. J. Biol. Macromol. 2016, 86, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Galus, S.; Lenart, A. Development and characterization of composite edible films based on sodium alginate and pectin. J. Food Eng. 2013, 115, 459–465. [Google Scholar] [CrossRef]
- Aloui, H.; Deshmukh, A.R.; Khomlaem, C.; Kim, B.S. Novel composite films based on sodium alginate and gallnut extract with enhanced antioxidant, antimicrobial, barrier and mechanical properties. Food Hydrocoll. 2020, 113, 106508. [Google Scholar] [CrossRef]
- Shahbazi, Y. The properties of chitosan and gelatin films incorporated with ethanolic red grape seed extract and Ziziphora clinopodioides essential oil as biodegradable materials for active food packaging. Int. J. Biol. Macromol. 2017, 99, 746–753. [Google Scholar] [CrossRef]
- Rubilar, J.F.; Cruz, R.M.S.; Silva, H.D.; Vicente, A.A.; Khmelinskii, I.; Vieira, M.C. Physico-mechanical properties of chitosan films with carvacrol and grape seed extract. J. Food Eng. 2013, 115, 466–474. [Google Scholar] [CrossRef] [Green Version]
- Han, Y.; Yu, M.; Wang, L. Physical and antimicrobial properties of sodium alginate/carboxymethyl cellulose films incorporated with cinnamon essential oil. Food Packag. Shelf Life 2018, 15, 35–42. [Google Scholar] [CrossRef]
- Ahmed, J.; Mulla, M.Z.; Arfat, Y.A. Thermo-mechanical, structural characterization and antibacterial performance of solvent casted polylactide/cinnamon oil composite films. Food Control. 2016, 69, 196–204. [Google Scholar] [CrossRef]
- Rambabu, K.; Bharath, G.; Banat, F.; Show, P.L.; Cocoletzi, H.H. Mango leaf extract incorporated chitosan antioxidant film for active food packaging. Int. J. Biol. Macromol. 2018, 126, 1234–1243. [Google Scholar] [CrossRef]
- Luís, Â.; Pereira, L.; Domingues, F.; Ramos, A. Development of a carboxymethyl xylan film containing licorice essential oil with antioxidant properties to inhibit the growth of foodborne pathogens. LWT Food Sci. Technol. 2019, 111, 218–225. [Google Scholar] [CrossRef]
- Mahcene, Z.; Khelil, A.; Hasni, S.; Akman, P.K.; Bozkurt, F.; Birech, K.; Goudjil, M.B.; Tornuk, F. Development and characterization of sodium alginate based active edible films incorporated with essential oils of some medicinal plants. Int. J. Biol. Macromol. 2019, 15, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Abugoch, L.E.; Tapia, C.; Villamán, M.C.; Yazdani-Pedram, M.; Díaz-Dosque, M. Characterization of quinoa protein–chitosan blend edible films. Food Hydrocoll. 2011, 25, 879–886. [Google Scholar] [CrossRef]
- Himejima, M.; Kubo, I. Antibacterial agents from the cashew Anacardium occidentale (Anacardiaceae) nut shell oil. J. Agric. Food Chem. 1991, 39, 418–421. [Google Scholar] [CrossRef]
- Branco, A.F.; Giallongo, F.; Frederick, T.; Weeks, H.; Oh, J.; Hristov, A.N. Effect of technical cashew nut shell liquid on rumen methane emission and lactation performance of dairy cows. J. Dairy Sci. 2015, 98, 4030–4040. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ngo, T.M.P.; Dang, T.M.Q.; Tran, T.X.; Rachtanapun, P. Effects of Zinc Oxide Nanoparticles on the Properties of Pectin/Alginate Edible Films. Int. J. Polym. Sci. 2018, 1, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Barreiros, A.L.B.S.; David, J.M.; David, J.P. Oxidative stress: Relations between the formation of reactive species and the organism’s defense. Quím. Nova 2006, 29, 113–123. [Google Scholar] [CrossRef] [Green Version]
- Voirin, C.; Caillol, S.; Sadavarte, N.V.; Tawade, B.V.; Boutevinab, B.; Wadgaonkar, P.P. Functionalization of cardanol: Towards biobased polymers and additives. Polym. Chem. 2014, 5, 3142–3162. [Google Scholar] [CrossRef]
- Kulisic, T.; Radonic, A.; Katalinic, V.; Milos, M. Use of different methods for testing antioxidative activity of oregano essential oil. Food Chem. 2004, 85, 633–640. [Google Scholar] [CrossRef]
- Benbettaïeb, N.; Mahfoudh, R.; Moundanga, S.; Brachais, C.H.; Chambin, O.; Debeaufort, F. Modeling of the release kinetics of phenolic acids embedded in gelatin/chitosan bioactive-packaging films: Influence of both water activity and viscosity of the food simulant on the film structure and antioxidant activity. Int. J. Biol. Macromolecules 2020, 160, 780–794. [Google Scholar] [CrossRef]
- Estevez-Areco, S.; Guz, L.; Famá, L.; Candal, R.; Goyanes, S. Bioactive starch nanocomposite films with antioxidant activity and enhanced mechanical properties obtained by extrusion followed by thermocompression. Food Hydrocoll. 2019, 96, 518–528. [Google Scholar] [CrossRef]
- Bastarrachea, L.J.; Wong, D.E.; Roman, M.J.; Lin, Z.; Goddard, J.M. Active packaging coatings. Coatings 2015, 5, 771–791. [Google Scholar] [CrossRef]
- Guissoni, A.C.P.; Silva, I.G.; Geris, R.; Cunha, L.C.; Silva, H.H.G. Larvicidal activity of Anacardium occidentale as an alternative to control Aedes aegypti and its toxicity in Rattus norvegicus. Rev. Bras. Pl. Med. 2013, 15, 363–367. [Google Scholar] [CrossRef] [Green Version]
- França, F.C.F.; Coelho, E.D.L.; Uchôa, A.F.J.; Rodrigues, F.H.A.; Ribeiro, M.E.N.P.; Soares, S.D.A.; Ricardo, N.M.P.S. Synthesis and characterization of alkylphenyl polyglycosidic surfactants from amylose and alkyl phenols extracted from natural CNSL. Quím. Nova 2016, 39, 771–781. [Google Scholar] [CrossRef]
- Osmari, M.P.; Matos, L.F.; Salab, B.L.; Diaz, T.G.; Giotto, F.M. Cashew nut shell liquid: Characteristics and applicability in animal production. Arq. Bras. Med. Vet. Zootec. 2015, 9, 143–149. [Google Scholar] [CrossRef] [Green Version]




| Variables | Cashew Nut Shell Liquid (%) | |||
|---|---|---|---|---|
| 0 | 0.5 | 1.0 | 1.5 | |
| Coloration index | ||||
| Luminosity (L*) | 91.59 ± 1.02 a | 80.80 ± 1.10 b | 65.83 ± 3.95 d | 61.57 ± 2.02 c |
| Redness (a*) | −1.10 ± 0.14 d | 1.61 ± 0.24 c | 6.73 ± 0.67 b | 9.34 ± 0.45 a |
| Yelowness (b*) | 7.01 ± 0.96 c | 16.9 ± 0.69 b | 24.00 ± 0.61 a | 24.03 ± 0.23 a |
| Chroma (C*) | 7.09 ± 0.97 c | 17.07 ± 0.70 b | 24.76 ± 0.63 a | 25.75 ± 0.31 a |
| Opaciy | 13.89 ± 0.34 d | 15.84 ± 0.91 c | 18.12 ± 0.75 b | 20.10 ± 0.98 a |
| Mechanical properties | ||||
| Thickness (mm) | 0.090 ± 0.02 b | 0.133± 0.04 a | 0.238± 0.04 a | 0.263±0.02 a |
| Tensile strength (MPa) | 54.71 ± 0.20 a | 41.54 ± 0.08 c | 44.03 ± 0.07 b | 36.58± 0.07 d |
| Modulus of elasticity (MPa) | 95.75 ± 0.36 a | 72.70 ± 0.14 c | 77.04 ± 0.12 b | 64.01± 0.13 d |
| Elongation at break (%) | 42.26 ± 3.94 b | 38.23 ± 2.94 b | 49.78 ± 4.67 a | 51.07 ± 1.88 a |
| WVP [10−10 g.(m.s.Pa)−1] | 7.16 ± 0.30 c | 9.11 ± 0.19 b | 29.12 ± 7.11 a | 31.02 ± 3.24 a |
| Strains | Cashew Nut Shell Liquid (µg/mL) | Azithromycin (µg/mL) | ||
|---|---|---|---|---|
| MIC | MBC | MIC | MBC | |
| S. typhimurium | 128 | 256 | 2 | 4 |
| E. coli | 512 | nd | 32 | 16 |
| P. aeruginosa | 128 | 256 | 8 | 16 |
| L. monocytogenes | 128 | 256 | 8 | 16 |
| B. cereus | 128 | 256 | 4 | 8 |
| S. aureus | 32 | 64 | 8 | 16 |
| Microorganisms | Filmogenic Solutions (CNSL) | Controls | ||||
|---|---|---|---|---|---|---|
| 0% | 0.5% | 1.0% | 1.5% | Azithromycin | Water | |
| S. typhimurium | NI | NI | NI | NI | 21.6 ± 0.57 | NI |
| E. coli | NI | NI | NI | NI | 16.3 ± 0.57 | NI |
| P. aeruginosa | NI | NI | 7.3 ± 1.15c | 10.6 ± 0.57b | 18.3 ± 0.57a | NI |
| L. monocytogenes | NI | NI | 7.5 ± 0.50c | 11.5 ± 0.50b | 18.5 ± 0.50a | NI |
| B. cereus | NI | NI | 6.5 ± 0.50b | 7.6 ± 0.5b | 18.6 ± 0.57a | NI |
| S. aureus | NI | NI | 9.6 ± 0.57c | 11.3 ± 0.57b | 20.3 ± 0.57a | NI |
| Sample | DPPH | ABTS | TAC |
|---|---|---|---|
| CNSL | 132.89 ± 0.27 a | 102.38 ± 0.31 b | 1482.81 ± 0.44 b |
| Trolox | 28.13 ± 0.11 b | 153.67 ± 0.02 a | N.T. |
| Ascorbic acid | N.T. | N.T. | 50000 ± 0.01 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vasconcelos, L.; de Souza, M.; de Oliveira, J.; Silva Filho, E.; Silva, A.; Mazzetto, S.E.; Pereira, E.S.; Oliveira, R.L.; Bezerra, L. Elaboration and Characterization of Bioactive Films Obtained from the Incorporation of Cashew Nut Shell Liquid into a Matrix of Sodium Alginate. Antioxidants 2021, 10, 1378. https://doi.org/10.3390/antiox10091378
Vasconcelos L, de Souza M, de Oliveira J, Silva Filho E, Silva A, Mazzetto SE, Pereira ES, Oliveira RL, Bezerra L. Elaboration and Characterization of Bioactive Films Obtained from the Incorporation of Cashew Nut Shell Liquid into a Matrix of Sodium Alginate. Antioxidants. 2021; 10(9):1378. https://doi.org/10.3390/antiox10091378
Chicago/Turabian StyleVasconcelos, Larruama, Marthyna de Souza, Juliana de Oliveira, Edson Silva Filho, André Silva, Selma Elaine Mazzetto, Elzânia Sales Pereira, Ronaldo Lopes Oliveira, and Leilson Bezerra. 2021. "Elaboration and Characterization of Bioactive Films Obtained from the Incorporation of Cashew Nut Shell Liquid into a Matrix of Sodium Alginate" Antioxidants 10, no. 9: 1378. https://doi.org/10.3390/antiox10091378