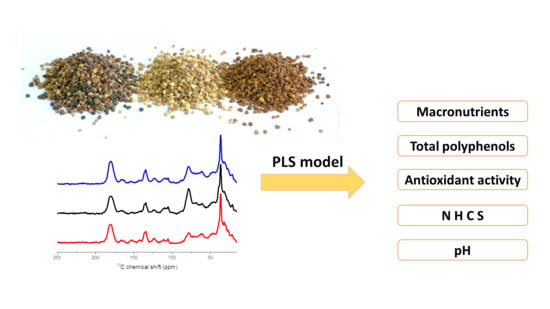
Modeling of Antioxidant Activity, Polyphenols and Macronutrients Content of Bee Pollen Applying Solid-State 13C NMR Spectra
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Material
2.2. Reference Analyzes
2.3. Chemicals and Reagents
2.4. Measurement Conditions
2.5. Multivariate Modeling
2.6. Software and Numerical Data Treatment
3. Results and Discussion
3.1. Solid-State NMR Spectra
3.2. Principal Component Analysis (PCA)
3.3. Construction of PLS Models
3.3.1. Nutrients Content Modeling
3.3.2. Modeling of Total Polyphenols Content and Antioxidant Activity
3.3.3. Modeling of Nitrogen, Carbon and Sulfur Content
3.3.4. Modeling of pH
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Denisow, B.; Denisow-Pietrzyk, M. Biological and therapeutic properties of bee pollen: A review. J. Sci. Food Agric. 2016, 96, 4303–4309. [Google Scholar] [CrossRef]
- Feas, X.; Vazquez-Tato, M.P.; Estevinho, L.; Seijas, J.A.; Iglesias, A. Organic bee pollen: Botanical origin, nutritional value, bioactive compounds, antioxidant activity and microbiological quality. Molecules 2012, 17, 8359–8377. [Google Scholar] [CrossRef]
- Thakur, M.; Nanda, V. Composition and functionality of bee pollen: A review. Trends Food Sci. Technol. 2020, 98, 82–106. [Google Scholar] [CrossRef]
- Almeida-Muradian, L.B.; Pamplona, L.C.; Coimbra, S.; Barth, O.M. Chemical composition and botanical evaluation of dried bee pollen pellets. J. Food. Comp. Anal. 2005, 18, 105–111. [Google Scholar] [CrossRef]
- Leja, M.; Mareczek, A.; Wyzgolik, G.; Klepacz-Baniak, J.; Czekonska, K. Antioxidative properties of bee pollen in selected plant species. Food Chem. 2007, 100, 237–240. [Google Scholar] [CrossRef]
- Rzepecka-Stojko, A.; Stojko, J.; Kurek-Gorecka, A.; Gorecki, M.; Kabala-Dzik, A.; Kubina, R.; Mozdzierz, A.; Buszman, E. Polyphenols from bee pollen: Structure, absorption, metabolism and biological activity. Molecules 2015, 20, 21732–21749. [Google Scholar] [CrossRef] [Green Version]
- Sattler, J.A.G.; de Melo, I.L.P.; Granato, D.; Araujo, E.; de Freitas, A.D.; Barth, O.M.; Sattler, A.; de Almeida-Muradian, L.B. Impact of origin on bioactive compounds and nutritional composition of bee pollen from southern Brazil: A screening study. Food Res. Int. 2015, 77, 82–91. [Google Scholar] [CrossRef] [Green Version]
- Cornara, L.; Biagi, M.; Xiao, J.B.; Burlando, B. Therapeutic properties of bioactive compounds from different honeybee products. Front. Pharmacol. 2017, 8, 412. [Google Scholar] [CrossRef]
- Hatzakis, E. Nuclear Magnetic Resonance (NMR) Spectroscopy in Food Science: A Comprehensive Review. Compr. Rev. Food Sci. F. 2019, 18, 189–220. [Google Scholar] [CrossRef] [Green Version]
- Bertocchi, F.; Paci, M. Applications of high-resolution solid-state NMR spectroscopy in food science. J. Agric. Food. Chem. 2008, 56, 9317–9327. [Google Scholar] [CrossRef] [PubMed]
- Parlak, Y.; Guzeler, N. Nuclear magnetic resonance spectroscopy applications in foods. Curr. Res. Nutr. Food Sci. 2016, 4, 161–168. [Google Scholar] [CrossRef]
- Ogrinc, N.; Kosir, I.J.; Spangenberg, J.E.; Kidric, J. The application of NMR and MS methods for detection of adulteration of wine, fruit juices, and olive oil. A review. Anal. Bioanal. Chem. 2003, 376, 424–430. [Google Scholar] [CrossRef] [PubMed]
- Santos, C.M.M.; Silva, A.M.S. Valuable analytical tools in analysis of honeybee plant-derived compounds: Nuclear magnetic resonance spectroscopy. In Chemistry, Biology and Potential Applications of Honeybee Plant Derived Products; Cardoso, S.M., Silva, A.M.S., Eds.; Bentham Science Publishers: Sharjah, United Arab Emirates, 2016; pp. 150–195. [Google Scholar]
- Siddiqui, A.J.; Musharraf, S.G.; Choudhary, M.I.; Rahman, A.U. Application of analytical methods in authentication and adulteration of honey. Food Chem. 2017, 217, 687–698. [Google Scholar] [CrossRef]
- Bertelli, D.; Lolli, M.; Papotti, G.; Bortolotti, L.; Serra, G.; Plessi, M. Detection of honey adulteration by sugar syrups using one-dimensional and two-dimensional high-resolution nuclear magnetic resonance. J. Agric. Food. Chem. 2010, 58, 8495–8501. [Google Scholar] [CrossRef]
- Zielinski, L.; Deja, S.; Jasicka-Misiak, I.; Kafarski, P. Chemometrics as a tool of origin determination of Polish monofloral and multifloral honeys. J. Agric. Food. Chem. 2014, 62, 2973–2981. [Google Scholar] [CrossRef]
- Kazalaki, A.; Misiak, M.; Spyros, A.; Dais, P. Identification and quantitative determination of carbohydrate molecules in Greek honey by employing 13C NMR spectroscopy. Anal. Methods 2015, 7, 5962–5972. [Google Scholar] [CrossRef]
- Mazzoni, V.; Bradesi, P.; Tomi, F.; Casanova, J. Direct qualitative and quantitative analysis of carbohydrate mixtures using 13C NMR spectroscopy: Application to honey. Magn. Reson. Chem. 1997, 35, S81–S90. [Google Scholar] [CrossRef]
- Kameda, T.; Tamada, Y. Variable-temperature 13C solid-state NMR study of the molecular structure of honeybee wax and silk. Int. J. Biol. Macromol. 2009, 44, 64–69. [Google Scholar] [CrossRef]
- Paradowska, K.; Zielinska, A.; Kuras, M.; Wawer, I. The composition of bee pollen color fractions evaluated by solid-state 1H and 13C NMR: Their macroelement content and antioxidant properties. J. Apic. Res. 2017, 56, 523–532. [Google Scholar] [CrossRef]
- Weglinska, M.; Szostak, R.; Kita, A.; Nems, A.; Mazurek, S. Determination of nutritional parameters of bee pollen by Raman and infrared spectroscopy. Talanta 2020, 212, 8. [Google Scholar] [CrossRef]
- Winning, H.; Larsen, F.H.; Bro, R.; Engelsen, S.B. Quantitative analysis of NMR spectra with chemometrics. J. Magn. Reson. 2008, 190, 26–32. [Google Scholar] [CrossRef]
- Hauksson, J.B.; Edlund, U.; Trygg, J. NMR processing techniques based on multivariate data analysis and orthogonal signal correction. 13C CP/MAS NMR spectroscopic characterization of softwood kraft pulp. Magn. Reson. Chem. 2001, 39, 267–275. [Google Scholar] [CrossRef]
- Engelsen, S.B.; Savorani, F.; Rasmussen, M.A. Chemometric exploration of quantitative NMR data. eMagRes 2013, 2, 267–278. [Google Scholar] [CrossRef]
- Smolinska, A.; Blanchet, L.; Buydens, L.M.C.; Wijmenga, S.S. NMR and pattern recognition methods in metabolomics: From data acquisition to biomarker discovery: A review. Anal. Chim. Acta 2012, 750, 82–97. [Google Scholar] [CrossRef]
- Breton, R.C.; Reynolds, W.F. Using NMR to identify and characterize natural products. Nat. Prod. Rep. 2013, 30, 501–524. [Google Scholar] [CrossRef]
- AOAC. (968.28) Sugars/Total Reducing Sugars. Titration Method. In Official Methods of Analysis of AOAC International, 16th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 1995. [Google Scholar]
- AOAC. (981.10) Nitrogen Content. Kjeldahl Method. In Official Methods of Analysis of AOAC International, 16th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 1995. [Google Scholar]
- AOAC. (936.15) Fat Content in Foods. Soxhlet Extraction Method. In Official Methods of Analysis of AOAC International, 16th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 1995. [Google Scholar]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Campos, M.G.R.; Bogdanov, S.; de Almeida-Muradian, L.B.; Szczesna, T.; Mancebo, Y.; Frigerio, C.; Ferreira, F. Pollen composition and standardisation of analytical methods. J. Apic. Res. 2008, 47, 154–161. [Google Scholar] [CrossRef]
- Fuenmayor, C.; Zuluaga, C.; Diaz, C.; Quiczan, D.; Cosio, M.; Mannino, S. Evaluation of the physicochemical and functional properties of Colombian bee pollen. Rev. MVZ Cordoba 2014, 19, 4003–4014. [Google Scholar] [CrossRef] [Green Version]
- Liolios, V.; Tananaki, C.; Dimou, M.; Kanelis, D.; Rodopoulou, M.A.; Thrasyvoulou, A. Exploring the sugar profile of unifloral bee pollen using high performance liquid chromatography. J. Food Nutri. Res. 2018, 57, 341–350. [Google Scholar]
- Habryka, C.; Socha, R.; Juszczak, L. Effect of bee pollen addition on the polyphenol content, antioxidant activity, and quality parameters of honey. Antioxidants 2021, 10, 810. [Google Scholar] [CrossRef]
- Martinello, M.; Mutinelli, F. Antioxidant Activity in Bee Products: A Review. Antioxidants 2021, 10, 71. [Google Scholar] [CrossRef]
- Barbieri, D.; Gabriele, M.; Summa, M.; Colosimo, R.; Leonardi, D.; Domenici, V.; Pucci, L. Antioxidant, nutraceutical properties, and fluorescence spectral profiles of bee pollen samples from different botanical origins. Antioxidants 2020, 9, 1001. [Google Scholar] [CrossRef]
- De-Melo, A.A.M.; Estevinho, L.M.; Moreira, M.M.; Delerue-Matos, C.; de Freitas, A.D.; Barth, O.M.; de Almeida-Muradian, L.B. A multivariate approach based on physicochemical parameters and biological potential for the botanical and geographical discrimination of Brazilian bee pollen. Food Biosc. 2018, 25, 91–110. [Google Scholar] [CrossRef] [Green Version]
- LeBlanc, B.W.; Davis, O.K.; Boue, S.; DeLucca, A.; Deeby, T. Antioxidant activity of Sonoran Desert bee pollen. Food Chem. 2009, 115, 1299–1305. [Google Scholar] [CrossRef]
- Oroian, M.; Ursachi, F.; Dranca, F. Ultrasound-assisted extraction of polyphenols from crude pollen. Antioxidants 2020, 9, 322. [Google Scholar] [CrossRef]
- Kocot, J.; Kielczykowska, M.; Luchowska-Kocot, D.; Kurzepa, J.; Musik, I. Antioxidant potential of propolis, bee pollen, and royal jelly: Possible medical application. Oxid. Med. Cell. Longev. 2018, 2018. [Google Scholar] [CrossRef] [PubMed]
- Pascoal, A.; Rodrigues, S.; Teixeira, A.; Feas, X.; Estevinho, L.M. Biological activities of commercial bee pollens: Antimicrobial, antimutagenic, antioxidant and anti-inflammatory. Food Chem. Toxicol. 2014, 63, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Pohl, P.; Dzimitrowicz, A.; Greda, K.; Jamroz, P.; Lesniewicz, A.; Szymczycha-Madeja, A.; Welna, M. Element analysis of bee-collected pollen and bee bread by atomic and mass spectrometry—Methodological development in addition to environmental and nutritional aspects. TrAC Trends Anal. Chem. 2020, 128, 115922. [Google Scholar] [CrossRef]
- Matuszewska, E.; Klupczynska, A.; Maciołek, K.; Kokot, Z.J.; Matysiak, J. Multielemental analysis of bee pollen, propolis, and royal jelly collected in west-central Poland. Molecules 2021, 26, 2415. [Google Scholar] [CrossRef]
- Filipiak, M.; Kuszewska, K.; Asselman, M.; Denisow, B.; Stawiarz, E.; Woyciechowski, M.; Weiner, J. Ecological stoichiometry of the honeybee: Pollen diversity and adequate species composition are needed to mitigate limitations imposed on the growth and development of bees by pollen quality. PLoS ONE 2017, 12, e0183236. [Google Scholar] [CrossRef]




| No. | Origin of Sample | TP a [mg GAE/g] | AOA b ABTS [µM TE/g] | Reducing sugars [%] | Protein [%] | Fat [%] | NHCS [%] | C [%] | N [%] | S [%] | pH |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Legnica | 12.33 | 168.5 | 27.6 | 22.38 | 9.64 | 56.10 | 45.09 | 3.58 | 0.105 | 4.05 |
| 2 | Legnica | 12.08 | 172.6 | 32.0 | 23.56 | 9.78 | 57.30 | 46.02 | 3.77 | 0.076 | 4.64 |
| 3 | Stróże | 12.54 | 223.1 | 41.0 | 25.00 | 7.58 | 57.51 | 46.13 | 4.00 | 0.112 | 4.55 |
| 4 | Stróże | 9.52 | 157.0 | 32.4 | 20.81 | 8.94 | 56.61 | 45.73 | 3.33 | 0.097 | 4.88 |
| 5 | Sulęcin | 11.46 | 178.6 | 29.6 | 24.13 | 10.20 | 58.11 | 46.65 | 3.86 | 0.061 | 4.66 |
| 6 | Łódź | 8.98 | 168.2 | 35.2 | 20.63 | 8.97 | 56.15 | 45.59 | 3.30 | 0.131 | 4.92 |
| 7 | Malbork | 7.91 | 149.2 | 42.2 | 17.69 | 8.95 | 56.04 | 45.87 | 2.83 | 0.103 | 4.59 |
| 8 | Otmuchów | 9.35 | 181.9 | 35.2 | 22.88 | 9.17 | 57.75 | 46.58 | 3.66 | 0.158 | 4.76 |
| 9 | Otmuchów | 8.11 | 160.7 | 36.4 | 19.75 | 7.89 | 56.22 | 45.63 | 3.16 | 0.125 | 4.96 |
| 10 | Stróże | 4.16 | 117.2 | 38.9 | 15.88 | 7.90 | 53.37 | 43.73 | 2.54 | 0.102 | 4.97 |
| 11 | Sokołów Małopolski | 13.01 | 235.3 | 42.8 | 25.63 | 7.76 | 57.98 | 46.52 | 4.10 | 0.162 | 4.74 |
| 12 | Wadowice | 7.69 | 167.0 | 39.6 | 16.94 | 7.84 | 55.95 | 45.34 | 2.71 | 0.118 | 5.59 |
| 13 | Uścikowo | 6.11 | 141.6 | 38.4 | 16.88 | 8.42 | 58.36 | 46.50 | 2.70 | 0.111 | 5.29 |
| 14 | Malbork | 9.41 | 160.8 | 40.8 | 15.69 | 7.87 | 56.66 | 45.92 | 2.51 | 0.180 | 5.29 |
| 15 | Suchlica | 7.12 | 152.9 | 44.8 | 26.19 | 8.38 | 57.69 | 46.75 | 4.19 | 0.202 | 4.54 |
| 16 | Łódź | 10.31 | 209.2 | 28.8 | 25.00 | 8.16 | 56.15 | 47.80 | 4.00 | 0.165 | 5.33 |
| 17 | Legnica | 9.70 | 180.7 | 39.2 | 21.88 | 11.16 | 58.72 | 44.73 | 3.50 | 0.143 | 4.84 |
| 18 | Częstochowa | 8.43 | 140.3 | 46.0 | 20.25 | 9.24 | 59.73 | 46.76 | 3.24 | 0.174 | 5.27 |
| 19 | Częstochowa | 10.61 | 208.6 | 34.7 | 25.50 | 7.56 | 55.56 | 46.13 | 4.08 | 0.165 | 5.25 |
| 20 | Wrocław | 7.80 | 155.7 | 36.3 | 21.06 | 8.66 | 58.46 | 45.10 | 3.37 | 0.145 | 4.96 |
| 21 | Stróże | 8.90 | 186.5 | 37.6 | 21.38 | 8.21 | 57.24 | 46.22 | 3.42 | 0.239 | 5.13 |
| 22 | Wola Węgierska | 10.32 | 172.7 | 37.9 | 21.13 | 8.26 | 55.93 | 46.86 | 3.38 | 0.172 | 4.86 |
| 23 | Bielsko-Biała | 9.84 | 190.6 | 37.9 | 21.75 | 8.04 | 57.37 | 46.05 | 3.48 | 0.123 | 4.81 |
| 24 | Rogóż | 8.15 | 169.8 | 37.0 | 20.00 | 10.01 | 57.45 | 46.46 | 3.20 | 0.160 | 4.83 |
| 25 | Otmuchów | 5.75 | 119.3 | 33.2 | 16.13 | 8.49 | 57.27 | 44.57 | 2.58 | 0.125 | 4.81 |
| 26 | Wambierzyce | 9.61 | 185.7 | 35.2 | 21.13 | 8.53 | 56.98 | 45.81 | 3.38 | 0.136 | 5.50 |
| 27 | Kozaki | 7.32 | 146.4 | 37.6 | 17.94 | 7.98 | 56.06 | 45.48 | 2.87 | 0.161 | 5.40 |
| 28 | Byków | 9.78 | 165.5 | 30.4 | 19.81 | 9.71 | 56.39 | 45.45 | 3.17 | 0.213 | 4.06 |
| 29 | Byków | 13.39 | 191.2 | 38.4 | 24.69 | 9.85 | 58.83 | 46.93 | 3.95 | 0.191 | 4.41 |
| 30 | Kamienna Góra | 14.57 | 264.8 | 40.8 | 28.81 | 8.25 | 59.37 | 47.47 | 4.61 | 0.192 | 5.80 |
| 31 | mix1 | 13.18 | 193.8 | 27.2 | 23.94 | 9.07 | 58.10 | 46.42 | 3.83 | 0.204 | 6.30 |
| 32 | mix2 | 13.92 | 210.8 | 23.2 | 27.63 | 12.28 | 58.92 | 46.72 | 4.42 | 0.228 | 4.74 |
| 33 | mix3 | 11.86 | 203.1 | 29.2 | 27.50 | 11.15 | 59.55 | 47.60 | 4.40 | 0.189 | 4.98 |
| 34 | mix4 | 12.84 | 196.3 | 25.6 | 25.13 | 11.98 | 59.31 | 47.32 | 4.02 | 0.227 | 5.14 |
| 35 | mix5 | 12.92 | 205.2 | 44.0 | 14.88 | 8.39 | 55.72 | 45.46 | 2.38 | 0.130 | 5.09 |
| Analyzed Feature | Parameter | |||||
|---|---|---|---|---|---|---|
| RSEPcal | RSEPval | R | Rcv | Number of LV | Pretreatment | |
| (Range) | [%] | [%] | ||||
| Reducing sugars (23.2–46.0%) | 2.37 | 2.38 | 0.991 | 0.908 | 4 | MSC |
| Protein (14.9–28.8%) | 2.02 | 2.52 | 0.992 | 0.899 | 4 | SNV |
| Fat (7.6–12.3%) | 1.79 | 2.22 | 0.991 | 0.862 | 4 | SNV |
| sum of NHCS (53.4–59.7%) | 0.47 | 0.62 | 0.975 | 0.766 | 5 | none |
| C (44.7–47.8%) | 0.32 | 0.42 | 0.981 | 0.780 | 5 | none |
| N (2.4–4.6%) | 1.85 | 1.89 | 0.994 | 0.922 | 4 | SNV |
| S (0.06–0.24%) | 4.23 | 6.07 | 0.992 | 0.768 | 5 | MSC |
| Total polyphenols (4.2–14.6 mg GAE/g) | 2.73 | 3.51 | 0.995 | 0.887 | 4 | MSC |
| Antioxidant activity ABTS (117.2–264.8 µM TE/g) | 2.80 | 3.83 | 0.985 | 0.881 | 3 | none |
| pH (4.1–5.8) | 1.43 | 2.14 | 0.994 | 0.777 | 7 | none |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazurek, S.; Szostak, R.; Kondratowicz, M.; Węglińska, M.; Kita, A.; Nemś, A. Modeling of Antioxidant Activity, Polyphenols and Macronutrients Content of Bee Pollen Applying Solid-State 13C NMR Spectra. Antioxidants 2021, 10, 1123. https://doi.org/10.3390/antiox10071123
Mazurek S, Szostak R, Kondratowicz M, Węglińska M, Kita A, Nemś A. Modeling of Antioxidant Activity, Polyphenols and Macronutrients Content of Bee Pollen Applying Solid-State 13C NMR Spectra. Antioxidants. 2021; 10(7):1123. https://doi.org/10.3390/antiox10071123
Chicago/Turabian StyleMazurek, Sylwester, Roman Szostak, Mateusz Kondratowicz, Magdalena Węglińska, Agnieszka Kita, and Agnieszka Nemś. 2021. "Modeling of Antioxidant Activity, Polyphenols and Macronutrients Content of Bee Pollen Applying Solid-State 13C NMR Spectra" Antioxidants 10, no. 7: 1123. https://doi.org/10.3390/antiox10071123