Use of Antioxidants for the Neuro-Therapeutic Management of COVID-19
Abstract
:1. Introduction
2. Pathophysiological Mechanisms of SARS-CoV-2 and Its Neurological Implications
3. Pharmacological Treatment for Patients with Neurological or Psychiatric Manifestations Associated with COVID-19
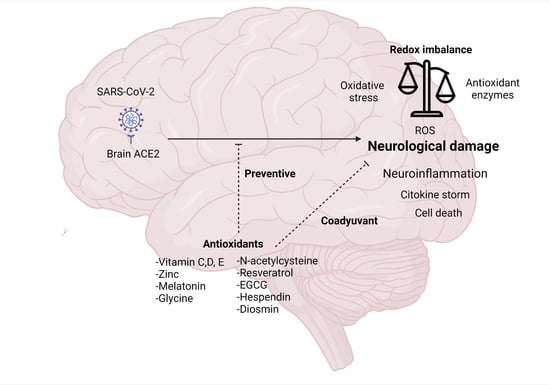
4. Role of Oxidative Stress and Antioxidant System in Patients with COVID-19
5. Mechanisms of Antioxidants Compounds against SARS-CoV-2 in COVID-19
6. Antioxidants as Neuroprotectors in Patients Infected with COVID-19
7. Perspectives
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization. WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int/ (accessed on 22 April 2021).
- Koyama, T.; Platt, D.; Parida, L. Variant analysis of SARS-cov-2 genomes. Bull. WHO 2020, 98, 495–504. [Google Scholar] [CrossRef]
- Ou, X.; Liu, Y.; Lei, X.; Li, P.; Mi, D.; Ren, L.; Guo, L.; Guo, R.; Chen, T.; Hu, J.; et al. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat. Commun. 2020, 11, 1620. [Google Scholar] [CrossRef] [Green Version]
- Dömling, A.; Gao, L. Chemistry and biology of SARS-CoV-2. Inside Chem. 2020, 6, 1283–1295. [Google Scholar] [CrossRef]
- Day, T.; Gandon, S.; Lion, S.; Otto, S.P. On the evolutionary epidemiology of SARS-CoV-2. Curr Biol. 2020, 30, R849–R857. [Google Scholar] [CrossRef] [PubMed]
- Lou, J.J.; Movassaghi, M.; Gordy, D.; Olson, M.G.; Zhang, T.; Khurana, M.S.; Chen, Z.; Perez-Rosendahl, M.; Thammachantha, S.; Singer, E.J.; et al. Neuropathology of COVID-19 (neuro-COVID): Clinicopathological update. Free Neuropathol. 2021, 18, 2. [Google Scholar] [CrossRef]
- Volz, E.; Hill, V.; McCrone, J.T.; Price, A.; Jorgensen, D.; O’Toole, Á.; Southgate, J.; Johnson, R.; Jackson, B.; Nascimento, F.F.; et al. Evaluating the Effects of SARS-CoV-2 Spike Mutation D614G on Transmissibility and Pathogenicity. Cell 2021, 7, 64–75.e11. [Google Scholar] [CrossRef] [PubMed]
- Naqvi, A.A.T.; Fatima, K.; Mohammad, T.; Fatima, U.; Singh, I.K.; Singh, A.; Atif, S.M.; Hariprasad, G.; Hasan, G.M.; Hassan, M.I. Insights into SARS-CoV-2 genome, structure, evolution, pathogenesis and therapies: Structural genomics approach. Biochim. Biophys. Acta Mol Basis Dis. 2020, 866, 165878. [Google Scholar] [CrossRef] [PubMed]
- Morawska, L.; Cao, J. Airborne transmission of SARS-CoV-2: The world should face the reality. Environ. Int. 2020, 139, 105730. [Google Scholar] [CrossRef]
- Ai, T.; Yang, Z.; Hou, H.; Zhan, C.; Chen, C.; Lv, W.; Tao, Q.; Sun, Z.; Xia, L. Correlation of Chest CT and RT-PCR Testing for Coronavirus Disease 2019 (COVID-19) in China: A Report of 1014 Cases. Radiology 2020, 296, E32–E40. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Wang, M.; Zhao, M.; Guo, S.; Xu, Y.; Ye, J.; Ding, W.; Wang, Z.; Ye, D.; Pan, W.; et al. The Clinical Characteristics and Prognosis Factors of Mild-Moderate Patients With COVID-19 in a Mobile Cabin Hospital: A Retrospective, Single-Center Study. Front. Public Health 2020, 5, 264. [Google Scholar] [CrossRef]
- Li, Q.; Guan, X.; Wu, P.; Wang, X.; Zhou, L.; Tong, Y.; Ren, R.; Leung, K.S.M.; Lau, E.H.Y.; Wong, J.Y.; et al. Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus-Infected Pneumonia. N. Engl. J. Med. 2020, 382, 1199–1207. [Google Scholar] [CrossRef] [PubMed]
- Hui, D.S.; Azhar, E.; Madani, T.A.; Ntoumi, F.; Kock, R.; Dar, O.; Ippolito, G.; Mchugh, T.D.; Memish, Z.A.; Drosten, C.; et al. The continuing 2019-nCoV epidemic threat of novel coronaviruses to global health—The latest 2019 novel coronavirus outbreak in Wuhan, China. Int. J. Infect. Dis. 2020, 91, 264–266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, N.; Zhou, M.; Dong, X.; Qu, J.; Gong, F.; Han, Y.; Qiu, Y.; Wang, J.; Liu, Y.; Wei, Y.; et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet 2020, 395, 507–513. [Google Scholar] [CrossRef] [Green Version]
- Pajo, A.T.; Espiritu, A.I.; Apor, A.; Jamora, R.D.G. Neuropathologic findings of patients with COVID-19: A systematic review. Neurol. Sci. 2021, 42, 1255–1266. [Google Scholar] [CrossRef]
- Domingues, R.B.; Mendes-Correa, M.C.; de Moura Leite, F.B.V.; Sabino, E.C.; Salarini, D.Z.; Claro, I.; Santos, D.W.; de Jesus, J.G.; Ferreira, N.E.; Romano, C.M.; et al. First case of SARS-COV-2 sequencing in cerebrospinal fluid of a patient with suspected demyelinating disease. J. Neurol. 2020, 267, 3154–3156. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Q.; Yang, Y.; Gao, J. Infectivity of human coronavirus in the brain. EBioMedicine 2020, 56, 102799. [Google Scholar] [CrossRef]
- Guedj, E.; Million, M.; Dudouet, P.; Tissot-Dupont, H.; Bregeon, F.; Cammilleri, S.; Raoult, D. 18F-FDG brain PET hypometabolism in post-SARS-CoV-2 infection: Substrate for persistent/delayed disorders? Eur. J. Nucl. Med. Mol. Imaging. 2021, 48, 592–595. [Google Scholar] [CrossRef]
- Pellegrini, L.; Albecka, A.; Mallery, D.L.; Kellner, M.J.; Paul, D.; Carter, A.P.; James, L.C.; Lancaster, M.A. SARS-CoV-2 Infects the Brain Choroid Plexus and Disrupts the Blood-CSF Barrier in Human Brain Organoids. Cell Stem. Cell 2020, 3, 951–961.e5. [Google Scholar] [CrossRef]
- Politi, L.S.; Salsano, E.; Grimaldi, M. Magnetic Resonance Imaging Alteration of the Brain in a Patient with Coronavirus Disease 2019 (COVID-19) and Anosmia. JAMA Neurol. 2020, 77, 1028–1029. [Google Scholar] [CrossRef]
- Bougakov, D.; Podell, K.; Goldberg, E. Multiple Neuroinvasive Pathways in COVID-19. Mol. Neurobiol. 2021, 58, 564–575. [Google Scholar] [CrossRef]
- Dubé, M.; Le Coupanec, A.; Wong, A.H.M.; Rini, J.M.; Desforges, M.; Talbot, P.J. Axonal Transport Enables Neuron-to-Neuron Propagation of Human Coronavirus OC43. J. Virol. 2018, 92, e00404-18. [Google Scholar] [CrossRef] [Green Version]
- Abboud, H.; Abboud, F.Z.; Kharbouch, H.; Arkha, Y.; El Abbadi, N.; El Ouahabi, A. COVID-19 and SARS-Cov-2 Infection: Pathophysiology and Clinical Effects on the Nervous System. World Neurosurg. 2020, 140, 49–53. [Google Scholar] [CrossRef]
- Miyazawa, M. Immunopathogenesis of SARS-CoV-2-induced pneumonia: Lessons from influenza virus infection. Inflamm. Regen. 2020, 40, 39. [Google Scholar] [CrossRef] [PubMed]
- Kantonen, J.; Mahzabin, S.; Mäyränpää, M.I.; Tynninen, O.; Paetau, A.; Andersson, N.; Sajantila, A.; Vapalahti, O.; Carpén, O.; Kekäläinen, E.; et al. Neuropathologic features of four autopsied COVID-19 patients. Brain Pathol. 2020, 30, 1012–1016. [Google Scholar] [CrossRef] [PubMed]
- Zhai, P.; Ding, Y.; Li, Y. The impact of COVID-19 on ischemic stroke. Diagn. Pathol. 2020, 15, 78. [Google Scholar] [CrossRef] [PubMed]
- Letko, M.; Marzi, A.; Munster, V. Functional assessment of cell entry and receptor usage for SARS-CoV-2 and other lineage B betacoronaviruses. Nat. Microbiol. 2020, 5, 562–569. [Google Scholar] [CrossRef] [Green Version]
- Fu, J.; Zhang, J.; Gong, Y.; Testa, C.L.; Klein-Szanto, A.J. Regulation of HIF-1 alpha by the proprotein convertases furin and PC7 in human squamous carcinoma cells. Mol. Carcinog. 2015, 54, 698–706. [Google Scholar] [CrossRef] [Green Version]
- Chauhan, A.; Kaur, R.; Chakrbarti, P.; Pal, A. "Silent Hypoxemia" Leads to Vicious Cycle of Infection, Coagulopathy and Cytokine Storm in COVID-19: Can Prophylactic Oxygen Therapy Prevent It? Indian J. Clin. Biochem. 2021, 15, 1–5. [Google Scholar] [CrossRef]
- Haga, S.; Yamamoto, N.; Nakai-Murakami, C.; Osawa, Y.; Tokunaga, K.; Sata, T.; Yamamoto, N.; Sasazuki, T.; Ishizaka, Y. Modulation of TNF-alpha-converting enzyme by the spike protein of SARS-CoV and ACE2 induces TNF-alpha production and facilitates viral entry. Proc. Natl. Acad. Sci. USA 2008, 105, 7809–7814. [Google Scholar] [CrossRef] [Green Version]
- Wu, D.; Yang, X.O. TH17 responses in cytokine storm of COVID-19: An emerging target of JAK2 inhibitor Fedratinib. J. Microbiol. Immunol. Infect. 2020, 53, 368–370. [Google Scholar] [CrossRef]
- Sun, S.H.; Chen, Q.; Gu, H.J.; Yang, G.; Wang, Y.X.; Huang, X.Y.; Liu, S.S.; Zhang, N.N.; Li, X.F.; Xiong, R.; et al. A Mouse Model of SARS-CoV-2 Infection and Pathogenesis. Cell Host Microbe 2020, 28, 124–133.e4. [Google Scholar] [CrossRef]
- Wang, J.; Jiang, M.; Chen, X.; Montaner, L.J. Cytokine storm and leukocyte changes in mild versus severe SARS-CoV-2 infection: Review of 3939 COVID-19 patients in China and emerging pathogenesis and therapy concepts. J. Leukoc. Biol. 2020, 108, 17–41. [Google Scholar] [CrossRef]
- Shenoy, S. Coronavirus (Covid-19) sepsis: Revisiting mitochondrial dysfunction in pathogenesis, aging, inflammation, and mortality. Inflamm. Res. 2020, 69, 1077–1085. [Google Scholar] [CrossRef] [PubMed]
- Nagu, P.; Parashar, A.; Behl, T.; Mehta, V. CNS implications of COVID-19: A comprehensive review. Rev. Neurosci. 2020, 32, 219–234. [Google Scholar] [CrossRef]
- Chen, K.H.; Wang, S.F.; Wang, S.Y.; Yang, Y.-P.; Wang, M.-L.; Chiou, S.-H.; Chang, Y.-L. Pharmacological development of the potential adjuvant therapeutic agents against coronavirus disease 2019. J Chin. Med. Assoc. 2020, 10, 1097. [Google Scholar] [CrossRef]
- Lan, J.; Ge, J.; Yu, J.; Shan, S.; Zhou, H.; Fan, S.; Zhang, Q.; Shi, X.; Wang, Q.; Zhang, L.; et al. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature 2020, 581, 215–220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belouzard, S.; Chu, V.C.; Whittaker, G.R. Activation of the SARS coronavirus spike protein via sequential proteolytic cleavage at two distinct sites. Proc. Natl. Acad. Sci. USA 2009, 106, 5871–5876. [Google Scholar] [CrossRef] [Green Version]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.-H.; Nitsche, A.; et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 2020, 181, 271–280. [Google Scholar] [CrossRef]
- Rodrigues Prestes, T.R.; Rocha, N.P.; Miranda, A.S.; Teixeira, A.L.; Simoes-E-Silva, A.C. The Anti-Inflammatory Potential of ACE2/Angiotensin-(1–7)/Mas Receptor Axis: Evidence from Basic and Clinical Research. Curr. Drug. Targets. 2017, 18, 1301–1313. [Google Scholar] [CrossRef]
- Bautista-Vargas, M.; Bonilla-Abadía, F.; Cañas, C.A. Potential role for tissue factor in the pathogenesis of hypercoagulability associated with in COVID-19. J. Thromb. Thrombolysis 2020, 1–5. [Google Scholar] [CrossRef]
- Yan, R.; Zhang, Y.; Li, Y.; Xia, L.; Guo, Y.; Zhou, Q. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science 2020, 367, 1444–1448. [Google Scholar] [CrossRef] [Green Version]
- Mody, V.; Ho, J.; Wills, S.; Mawri, A.; Lawson, L.; Ebert, M.C.C.J.C.; Fortin, G.M.; Rayalam, S.; Taval, S. Identification of 3-chymotrypsin like protease (3CLPro) inhibitors as potential anti-SARS-CoV-2 agents. Commun. Biol. 2021, 4, 93. [Google Scholar] [CrossRef]
- Lanza, K.; Perez, L.G.; Costa, L.B.; Cordeiro, T.M.; Palmeira, V.A.; Ribeiro, V.T.; Samoes e Silva, A.C. Covid-19: The renin-angiotensin system imbalance hypothesis. Clin. Sci. 2020, 134, 1259–1264. [Google Scholar] [CrossRef]
- Cantero-Navarro, E.; Fernández-Fernández, B.; Ramos, A.M.; Rayego-Mateos, S.; Rodrigues-Diez, R.R.; Sánchez-Niño, M.D.; Sanz, A.B.; Ruiz-Ortega, M.; Ortiz, A. Renin-angiotensin system and inflammation update. Mol. Cell. Endocrinol. 2021, 111254. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Yu, Y.; Yu, C. Antifibrotic Roles of RAAS Blockers: Update. Adv. Exp. Med. Biol. 2019, 1165, 671–691. [Google Scholar] [CrossRef] [PubMed]
- Hikmet, F.; Méar, L.; Edvinsson, Å.; Micke, P.; Uhlén, M.; Lindskog, C. The protein expression profile of ACE2 in human tissues. Mol. Syst. Biol. 2020, 16, e9610. [Google Scholar] [CrossRef]
- Chen, R.; Wang, K.; Yu, J.; Howard, D.; French, L.; Chen, Z.; Wen, C.; Xu, Z. The Spatial and Cell-Type Distribution of SARS-CoV-2 Receptor ACE2 in the Human and Mouse Brains. Front. Neurol. 2021, 20, 573095. [Google Scholar] [CrossRef]
- Dean, A.Q.; Bozza, W.P.; Twomey, J.D.; Luo, S.; Nalli, A.; Zhang, B. The fight against COVID-19: Striking a balance in the renin-angiotensin system. Drug. Discov. Today 2021. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Revelles, S.; Avendaño, M.S.; García-Redondo, A.B.; Alvarez, Y.; Aguado, A.; Pérez-Girón, J.V.; García-Redondo, L.; Esteban, V.; Redondo, J.M.; Alonso, M.J.; et al. Reciprocal relationship between reactive oxygen species and cyclooxygenase-2 and vascular dysfunction in hypertension. Antioxid. Redox. Signal. 2013, 18, 51–65. [Google Scholar] [CrossRef] [PubMed]
- Vajapey, R.; Rini, D.; Walston, J.; Abadir, P. The impact of age-related dysregulation of the angiotensin system on mitochondrial redox balance. Front. Physiol. 2014, 5, 439. [Google Scholar] [CrossRef] [Green Version]
- Tay, M.Z.; Poh, C.M.; Rénia, L.; MacAry, P.A.; Ng, L.F.P. The trinity of COVID-19: Immunity, inflammation and intervention. Nat. Rev. Immunol. 2020, 20, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Ruan, Q.; Yang, K.; Wang, W.; Jiang, L.; Song, J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Int. Care Med. 2020, 46, 846–848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.C.; Bai, W.Z.; Hashikawa, T. The neuroinvasive potential of SARS-CoV2 may play a role in the respiratory failure of COVID-19 patients. J. Med. Virol. 2020, 92, 552–555. [Google Scholar] [CrossRef] [PubMed]
- The Human Protein Atlas. Available online: https://www.proteinatlas.org/ENSG00000197635-DPP4/tissue (accessed on 2 June 2021).
- De Wit, E.; van Doremalen, N.; Falzarano, D.; Munster, V.J. SARS and MERS: Recent insights into emerging coronaviruses. Nat. Rev. Microbiol. 2016, 14, 523–534. [Google Scholar] [CrossRef]
- Lukiw, W.J.; Pogue, A.; Hill, J.M. SARS-CoV-2 Infectivity and Neurological Targets in the Brain. Cell Mol. Neurobiol. 2020, 25, 1–8. [Google Scholar] [CrossRef]
- Pennisi, M.; Lanza, G.; Falzone, L.; Fisicaro, F.; Ferri, R.; Bella, R. SARS-CoV-2 and the Nervous System: From Clinical Features to Molecular Mechanisms. Int. J. Mol. Sci. 2020, 21, 5475. [Google Scholar] [CrossRef]
- Kumari, P.; Rothan, H.A.; Natekar, J.P.; Stone, S.; Pathak, H.; Strate, P.G.; Arora, K.; Brinton, M.A.; Kumar, M. Neuroinvasion and Encephalitis Following Intranasal Inoculation of SARS-CoV-2 in K18-hACE2 Mice. Viruses 2021, 13, 132. [Google Scholar] [CrossRef]
- El Bini Dhouib, I. Does coronaviruses induce neurodegenerative diseases? A systematic review on the neurotropism and neuroinvasion of SARS-CoV-2. Drug. Discov. Ther. 2021, 14, 262–272. [Google Scholar] [CrossRef]
- Wu, Y.; Xu, X.; Chen, Z.; Duan, J.; Hashimoto, K.; Yang, L.; Liu, C.; Yang, C. Nervous system involvement after infection with COVID-19 and other coronaviruses. Brain. Behav. Immun. 2020, 87, 18–22. [Google Scholar] [CrossRef]
- Toscano, G.; Palmerini, F.; Ravaglia, S.; Ruiz, L.; Invernizzi, P.; Cuzzoni, M.G.; Franciotta, D.; Baldanti, F.; Daturi, R.; Postorino, P.; et al. Guillain-Barré Syndrome Associated with SARS-CoV-2. N. Engl. J. Med. 2020, 382, 2574–2576. [Google Scholar] [CrossRef]
- Kabbani, N.; Olds, J.L. Does COVID19 Infect the Brain? If So, Smokers Might Be at a Higher Risk. Mol. Pharmacol. 2020, 97, 351–353. [Google Scholar] [CrossRef]
- Brann, D.; Tsukahara, T.; Weinreb, C.; Lipovsek, M.; Van den Berge, K.; Gong, B.; Chance, R.; Macaulay, I.; Chou, H.-J.; Fletcher, R.B.; et al. Non-neuronal expression of SARS-CoV-2 entry genes in the olfactory system suggests mechanisms underlying COVID-19-associated anosmia. Sci. Adv. 2020, 6, eabc5801. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, F.A.A.; Palmeira, D.C.C.; Rocha-Filho, P.A.S. Headache and pleocytosis in CSF associated with COVID-19: Case report. Neurol. Sci. 2020, 11, 3021–3022. [Google Scholar] [CrossRef] [PubMed]
- Ellul, M.A.; Benjamin, L.; Singh, B.; Lant, S.; Michael, B.D.; Easton, A.; Kneen, R.; Defres, S.; Sejvar, J.; Solomon, T. Neurological associations of COVID-19. Lancet Neurol. 2020, 9, 767–783. [Google Scholar] [CrossRef]
- Suresh Kumar, V.C.; Mukherjee, S.; Harne, P.S.; Subedi, A.; Ganapathy, M.K.; Patthipati, V.S.; Sapkota, B. Novelty in the gut: A systematic review and meta-analysis of the gastrointestinal manifestations of COVID-19. BMJ Open Gastroenterol. 2020, 1, e000417. [Google Scholar] [CrossRef]
- Lippi, A.; Domingues, R.; Setz, C.; Outeiro, T.F.; Krisko, A. SARS-CoV-2: At the Crossroad Between Aging and Neurodegeneration. Mov. Disord. 2020, 5, 716–720. [Google Scholar] [CrossRef] [Green Version]
- Helms, J.; Kremer, S.; Merdji, H.; Clere-Jehl, R.; Schenck, M.; Kummerlen, C.; Collange, O.; Boulay, C.; Fafi-Kremer, S.; Ohana, M.; et al. Neurologic Features in Severe SARS-CoV-2 Infection. N. Engl. J. Med. 2020, 382, 2268–2270. [Google Scholar] [CrossRef] [PubMed]
- Montalvan, V.; Lee, J.; Bueso, T.; De Toledo, J.; Rivas, K. Neurological manifestations of COVID-19 and other coronavirus infections: A systematic review. Clin. Neurol. Neurosurg. 2020, 194, 105921. [Google Scholar] [CrossRef]
- Di Carlo, D.T.; Montemurro, N.; Petrella, G.; Siciliano, G.; Ceravolo, R.; Perrini, P. Exploring the clinical association between neurological symptoms and COVID-19 pandemic outbreak: A systematic review of current literature. J. Neurol. 2020, 268, 1561–1569. [Google Scholar] [CrossRef]
- Mao, L.; Jin, H.; Wang, M.; Hu, Y.; Chen, S.; He, Q.; Chang, J.; Hong, C.; Zhou, Y.; Wang, D.; et al. Neurologic Manifestations of Hospitalized Patients with Coronavirus Disease 2019 in Wuhan, China. JAMA Neurol. 2020, 77, 683–690. [Google Scholar] [CrossRef] [Green Version]
- Kholin, A.A.; Zavadenko, N.N.; Nesterovskiy, Y.E.; Kholina, E.A.; Zavadenko, A.N.; Khondkaryan, G.S. [Features of neurological manifestations of the COVID-19 in children and adults]. Zh. Nevrol. Psikhiatr. Im. S.S. Korsakova 2020, 120, 114–120. [Google Scholar] [CrossRef]
- Nath, A.; Smith, B. Neurological complications of COVID-19: From bridesmaid to bride. Arq. Neuropsiquiatr. 2020, 78, 459–460. [Google Scholar] [CrossRef]
- Fisicaro, F.; Di Napoli, M.; Liberto, A.; Fanella, M.; Di Stasio, F.; Pennisi, M.; Bella, R.; Lanza, G.; Mansueto, G. Neurological Sequelae in Patients with COVID-19: A Histopathological Perspective. Int. J. Environ. Res. Public Health 2021, 18, 1415. [Google Scholar] [CrossRef]
- Abdelnaby, R.; Elsayed, M.; Abele-Haupts, F.; Barkin, M.E.; Rudek, M.A.; Schmidt, K. COVID-19 induced encephalopathy—A Case Report. Res. Sq. 2020. [Google Scholar] [CrossRef]
- Abdi, S.; Ghorbani, A.; Fatehi, F. The association of SARS-CoV-2 infection and acute disseminated encephalomyelitis without prominent clinical pulmonary symptoms. J. Neurol. Sci. 2020, 416, 117001. [Google Scholar] [CrossRef]
- Anzalone, N.; Castellano, A.; Scotti, R.; Scandroglio, A.M.; Filippi, M.; Ciceri, F.; Tresoldi, M.; Falini, A. Multifocal laminar cortical brain lesions: A consistent MRI finding in neuro-COVID-19 patients. J. Neurol. 2020, 267, 2806–2809. [Google Scholar] [CrossRef] [PubMed]
- Conto-Palomino, N.M.; Cabrera-Bueno, M.L.; Vargas-Ponce, K.G.; Rondón-Abuhadba, E.A.; Atamari-Anahui, N. Encefalitis asociada a COVID-19 en una niña de 13 años: Reporte de caso [Encephalitis associated with COVID-19 in a 13-year-old girl: A case report]. Medwave 2020, 20, e7984. [Google Scholar] [CrossRef] [PubMed]
- Delorme, C.; Paccoud, O.; Kas, A.; Hesters, A.; Bombois, S.; Shambrook, P.; Boullet, A.; Doukhi, D.; Le Guennec, L.; Godefroy, N.; et al. COVID-19-related encephalopathy: A case series with brain FDG-positron-emission tomography/computed tomography findings. Eur. J. Neurol. 2020, 27, 2651–2657. [Google Scholar] [CrossRef] [PubMed]
- Espinosa, P.S.; Rizvi, Z.; Sharma, P.; Hindi, F.; Filatov, A. Neurological Complications of Coronavirus Disease (COVID-19): Encephalopathy, MRI Brain and Cerebrospinal Fluid Findings: Case 2. Cureus 2020, 12, e7930. [Google Scholar] [CrossRef]
- Filatov, A.; Sharma, P.; Hindi, F.; Espinosa, P.S. Neurological Complications of Coronavirus Disease (COVID-19): Encephalopathy. Cureus 2020, 12, e7352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freire-Álvarez, E.; Guillén, L.; Lambert, K.; Baidez, A.; García-Quesada, M.; Andreo, M.; Alom, J.; Masiá, M.; Gutiérrez, F. COVID-19-associated encephalitis successfully treated with combination therapy. Clin. Infect. Pract. 2020, 100053. [Google Scholar] [CrossRef]
- Goodloe, T.B. 3rd, Walter LA. COVID-19 Presenting as Encephalopathy in the Emergency Department: A Case Report. Clin. Pract. Cases Emerg. Med. 2021, 5, 26–29. [Google Scholar] [CrossRef]
- Hayashi, M.; Sahashi, Y.; Baba, Y.; Okura, H.; Shimohata, T. COVID-19-associated mild encephalitis/encephalopathy with a reversible splenial lesion. J. Neurol. Sci. 2020, 415, 116941. [Google Scholar] [CrossRef]
- Langley, L.; Zeicu, C.; Whitton, L.; Pauls, M. Acute disseminated encephalomyelitis (ADEM) associated with COVID-19. BMJ Case Rep. 2020, 13, e239597. [Google Scholar] [CrossRef]
- Muccioli, L.; Pensato, U.; Bernabè, G.; Ferri, L.; Tappatà, M.; Volpi, L.; Cani, I.; Henry, O.J.; Ceccaroni, F.; Cevoli, S.; et al. Intravenous immunoglobulin therapy in COVID-19-related encephalopathy. J. Neurol. 2020, 1–5. [Google Scholar] [CrossRef]
- Pensato, U.; Muccioli, L.; Pasini, E.; Tappatà, M.; Ferri, L.; Volpi, L.; Licchetta, L.; Battaglia, S.; Rossini, G.; Bon, I.; et al. Encephalopathy in COVID-19 Presenting with Acute Aphasia Mimicking Stroke. Front. Neurol. 2020, 11, 587226. [Google Scholar] [CrossRef] [PubMed]
- Sattar, S.B.A.; Haider, M.A.; Zia, Z.; Niazi, M.; Iqbal, Q.Z. Clinical, Radiological, and Molecular Findings of Acute Encephalitis in a COVID-19 Patient: A Rare Case Report. Cureus 2020, 12, e10650. [Google Scholar] [CrossRef] [PubMed]
- Vandervorst, F.; Guldolf, K.; Peeters, I.; Vanderhasselt, T.; Michiels, K.; Berends, K.J.; Van Laethem, J.; Pipeleers, L.; Vincken, S.; Seynaeve, L.; et al. Encephalitis associated with the SARS-CoV-2 virus: A case report. Interdiscip. Neurosurg. 2020, 22, 100821. [Google Scholar] [CrossRef] [PubMed]
- Virhammar, J.; Kumlien, E.; Fällmar, D.; Frithiof, R.; Jackmann, S.; Sköld, M.K.; Kadir, M.; Frick, J.; Lindeberg, J.; Olivero-Reinius, H.; et al. Acute necrotizing encephalopathy with SARS-CoV-2 RNA confirmed in cerebrospinal fluid. Neurology 2020, 95, 445–449. [Google Scholar] [CrossRef] [PubMed]
- Pizzanelli, C.; Milano, C.; Canovetti, S.; Tagliaferri, E.; Turco, F.; Verdenelli, S.; Nesti, L.; Franchi, M.; Bonanni, E.; Menichetti, F.; et al. Autoimmune limbic encephalitis related to SARS-CoV-2 infection: Case-report and review of the literature. Brain Behav. Immun. Health 2021, 12, 100210. [Google Scholar] [CrossRef]
- Grimaldi, S.; Lagarde, S.; Harlé, J.R.; Boucraut, J.; Guedj, E. Autoimmune Encephalitis Concomitant with SARS-CoV-2 Infection: Insight from 18F-FDG PET Imaging and Neuronal Autoantibodies. J. Nucl. Med. 2020, 61, 1726–1729. [Google Scholar] [CrossRef]
- Khodamoradi, Z.; Hosseini, S.A.; Gholampoor Saadi, M.H.; Mehrabi, Z.; Sasani, M.R.; Yaghoubi, S. COVID-19 meningitis without pulmonary involvement with positive cerebrospinal fluid PCR. Eur. J. Neurol. 2020, 27, 2668–2669. [Google Scholar] [CrossRef] [PubMed]
- Espíndola, O.M.; Siqueira, M.; Soares, C.N.; Lima, M.A.S.D.; Leite, A.C.C.B.; Araujo, A.Q.C.; Brandão, C.O.; Silva, M.T.T. Patients with COVID-19 and neurological manifestations show undetectable SARS-CoV-2 RNA levels in the cerebrospinal fluid. Int. J. Infect. Dis. 2020, 96, 567–569. [Google Scholar] [CrossRef] [PubMed]
- Varatharaj, A.; Thomas, N.; Ellul, M.A.; Davies, N.W.S.; Pollak, T.A.; Tenorio, E.L.; Sultan, M.; Easton, A.; Breen, G.; Zandi, M.; et al. Neurological and neuropsychiatric complications of COVID-19 in 153 patients: A UK-wide surveillance study. Lancet Psychiatry 2020, 7, 875–882. [Google Scholar] [CrossRef]
- Islam, M.R.; Rahman, T.; Ahmed, S.; Khan, M.S.; Azad, M.R.; Alam, D.; Habib, R. Neurological presentation of COVID-19: Experience from a tertiary care hospital of Bangladesh. BIRDEM Med. J. 2020, 10, 33–40. [Google Scholar] [CrossRef]
- Khedr, E.M.; Abo-Elfetoh, N.; Deaf, E.; Hassan, H.M.; Amin, M.T.; Soliman, R.K.; Atta, A.A.; Zarzour, A.A.; Zain, M.; Mohamed-Hussein, A.; et al. Surveillance Study of Acute Neurological Manifestations among 439 Egyptian Patients with COVID-19 in Assiut and Aswan University Hospitals. Neuroepidemiology 2021, 55, 109–118. [Google Scholar] [CrossRef]
- Andrea, M.; Christian, M.; Lorenzo, M.; Francesco, D.; Walter, A.; Marco, M.; Andreina, B.; Maria, G.; Paolo, G.; Daniela, D.; et al. Unusual Presentation of COVID-19: Encephalitis and Syndrome of Inappropriate Anti-Diuretic Hormone Secretion. Int. J. Clin. Med. 2020, 11, 559–564. [Google Scholar] [CrossRef]
- Tristán-Samaniego, D.P.; Chiquete, E.; Treviño-Frenk, I.; Rubalcava-Ortega, J.; Higuera-Calleja, J.A.; Romero-Sánchez, G.; Espinoza-Alvarado, L.; Barrera-Vargas, A.; Flores-Silva, F.; González-Duarte, A.; et al. COVID-19-related diffuse posthypoxic leukoencephalopathy and microbleeds masquerades as acute necrotizing encephalopathy. Int. J. Neurosci 2020, 1–6. [Google Scholar] [CrossRef]
- Rajdev, K.; Victor, N.; Buckholtz, E.S.; Hariharan, P.; Saeed, M.A.; Hershberger, D.M.; Bista, S.A. Case of Guillain-Barré Syndrome Associated with COVID-19. J. Investig. Med. High Impact. Case Rep. 2020, 8, 2324709620961198. [Google Scholar] [CrossRef]
- Chan, J.L.; Ebadi, H.; Sarna, J.R. Guillain-Barré Syndrome with Facial Diplegia Related to SARS-CoV-2 Infection. Can. J. Neurol. Sci. 2020, 47, 852–854. [Google Scholar] [CrossRef]
- Juliao Caamaño, D.S.; Alonso Beato, R. Facial diplegia, a possible atypical variant of Guillain-Barré Syndrome as a rare neurological complication of SARS-CoV-2. J. Clin. Neurosci. 2020, 77, 230–232. [Google Scholar] [CrossRef]
- Colonna, S.; Sciumé, L.; Giarda, F.; Innocenti, A.; Beretta, G.; Dalla Costa, D. Case Report: Postacute Rehabilitation of Guillain-Barré Syndrome and Cerebral Vasculitis-Like Pattern Accompanied by SARS-CoV-2 Infection. Front. Neurol. 2021, 11, 602554. [Google Scholar] [CrossRef]
- Fadakar, N.; Ghaemmaghami, S.; Masoompour, S.M.; Shirazi Yeganeh, B.; Akbari, A.; Hooshmandi, S.; Ostovan, V.R. A First Case of Acute Cerebellitis Associated with Coronavirus Disease (COVID-19): A Case Report and Literature Review. Cerebellum 2020, 19, 911–914. [Google Scholar] [CrossRef]
- Taylor, L.D.; Ameen, O.S.; Zaharie, S.D. Complete Clinicopathological Case Report of a Young Patient Dying of COVID-19-Related Stroke. Am. J. Forensic. Med. Pathol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Elhassan, M.; Saidahmed, O.; Adebayo, A.; Archibald, N. Persistent Cortical Blindness Following Posterior Reversible Encephalopathy Syndrome (PRES) as a Complication of COVID-19 Pneumonia. Cureus 2021, 13, e12794. [Google Scholar] [CrossRef]
- Princiotta Cariddi, L.; Tabaee Damavandi, P.; Carimati, F.; Banfi, P.; Clemenzi, A.; Marelli, M.; Giorgianni, A.; Vinacci, G.; Mauri, M.; Versino, M. Reversible Encephalopathy Syndrome (PRES) in a COVID-19 patient. J. Neurol. 2020, 267, 3157–3160. [Google Scholar] [CrossRef]
- Cavalcanti, D.D.; Raz, E.; Shapiro, M.; Dehkharghani, S.; Yaghi, S.; Lillemoe, K.; Nossek, E.; Torres, J.; Jain, R.; Riina, H.A.; et al. Cerebral Venous Thrombosis Associated with COVID-19. AJNR. Am. J. Neuroradiol. 2020, 41, 1370–1376. [Google Scholar] [CrossRef] [PubMed]
- Fu, B.; Chen, Y.; Li, P. Novel coronavirus disease with secondary ischemic stroke: Two case reports. BMC Neurol. 2021, 21, 4. [Google Scholar] [CrossRef]
- Kadono, Y.; Nakamura, Y.; Ogawa, Y.; Yamamoto, S.; Kajikawa, R.; Nakajima, Y.; Matsumoto, M.; Kishima, H. A case of COVID-19 infection presenting with a seizure following severe brain edema. Seizure 2020, 80, 53–55. [Google Scholar] [CrossRef] [PubMed]
- Klein, D.E.; Libman, R.; Kirsch, C.; Arora, R. Cerebral venous thrombosis: A typical presentation of COVID-19 in the young. J. Stroke Cerebrovasc. Dis. 2020, 29, 104989. [Google Scholar] [CrossRef] [PubMed]
- Oxley, T.J.; Mocco, J.; Majidi, S.; Kellner, C.P.; Shoirah, H.; Singh, I.P.; De Leacy, R.A.; Shigematsu, T.; Ladner, T.R.; Yaeger, K.A.; et al. Large-Vessel Stroke as a Presenting Feature of Covid-19 in the Young. N. Engl. J. Med. 2020, 382, e60. [Google Scholar] [CrossRef]
- Prasad, A.; Kataria, S.; Srivastava, S.; Lakhani, D.A.; Sriwastava, S. Multiple embolic stroke on magnetic resonance imaging of the brain in a COVID-19 case with persistent encephalopathy. Clin. Imaging 2021, 69, 285–288. [Google Scholar] [CrossRef] [PubMed]
- Rudilosso, S.; Esteller, D.; Urra, X.; Chamorro, Á. Thalamic perforating artery stroke on computed tomography perfusion in a patient with coronavirus disease 2019. J. Stroke Cerebrovasc. Dis. 2020, 29, 104974. [Google Scholar] [CrossRef]
- Saitta, L.; Molin, A.; Villani, F.; Insorsi, A.; Roccatagliata, L.; Inglese, M.; Bassetti, M.; Pelosi, P.; Castellan, L.; Gerevini, S.; et al. Brain microvascular occlusive disorder in COVID-19: A case report. Neurol. Sci. 2020, 41, 3401–3404. [Google Scholar] [CrossRef] [PubMed]
- Shawkat, A.; Merrell, E.T.; Fadel, G.A.; Amzuta, I.; Amin, H.; Shah, A.J.; Habeb, H.; Aiash, H. Multiple Thrombotic Events in a 67-Year-Old Man 2 Weeks After Testing Positive for SARS-CoV-2: A Case Report. Am. J. Case. Rep. 2020, 21, e925786. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, Y.; Tsuchiya, T.; Tanaka, R.; Ouchi, A.; Motoyama, A.; Takamoto, T.; Hara, N.; Yanagawa, Y. Cerebral venous thrombosis in COVID-19-associated coagulopathy: A case report. J. Clin. Neurosci. 2020, 79, 30–32. [Google Scholar] [CrossRef] [PubMed]
- Tunç, A.; Ünlübaş, Y.; Alemdar, M.; Akyüz, E. Coexistence of COVID-19 and acute ischemic stroke report of four cases. J. Clin. Neurosci. 2020, 77, 227–229. [Google Scholar] [CrossRef]
- Ahmad, H.; Shubair, S.M.; Kruer, J.; Hatoum, C.A. Acute Lower-Extremity Ischemia in a Patient with COVID-19. Am. J. Case Rep. 2021, 22, e928471. [Google Scholar] [CrossRef]
- Yeganegi, M.; Fattahi, P. Management and Prevention of Cerebrovascular Accidents in SARS-CoV-2-Positive Patients Recovering from COVID-19: A Case Report and Review of Literature. SN Compr. Clin. Med. 2021, 1–12. [Google Scholar] [CrossRef]
- Flores, G.; Kumar, J.I.; Pressman, E.; Sack, J.; Alikhani, P. Spontaneous Brainstem Hemorrhagic Stroke in the Setting of Novel Coronavirus Disease 2019—A Case Report. Cureus 2020, 12, e10809. [Google Scholar] [CrossRef]
- Keller, E.; Brandi, G.; Winklhofer, S.; Imbach, L.L.; Kirschenbaum, D.; Frontzek, K.; Steiger, P.X.; Dietlerx, S.; Haeberlin, M.; Willms, J.; et al. Large and Small Cerebral Vessel Involvement in Severe COVID-19: Detailed Clinical Workup of a Case Series. Stroke 2020, 51, 3719–3722. [Google Scholar] [CrossRef] [PubMed]
- Nawabi, J.; Morotti, A.; Wildgruber, M.; Boulouis, G.; Kraehling, H.; Schlunk, F.; Can, E.; Kniep, H.; Thomalla, G.; Psychogios, M.; et al. Clinical and Imaging Characteristics in Patients with SARS-CoV-2 Infection and Acute Intracranial Hemorrhage. J. Clin. Med. 2020, 9, 2543. [Google Scholar] [CrossRef] [PubMed]
- Abouhashem, S.; Eldawoody, H.; Taha, M.M. Cerebral venous sinus thrombosis in patients with COVID-19 infection. Interdiscip. Neurosurg. 2021, 24, 101091. [Google Scholar] [CrossRef] [PubMed]
- Thibodeau, R.; Jafroodifar, A.; Quraeshi, S.; Lisi, M. SARS-CoV-2 infection leading to ischemic and hemorrhagic brain lesions and acute respiratory distress syndrome. Radiol. Case. Rep. 2021, 16, 753–759. [Google Scholar] [CrossRef] [PubMed]
- Caronna, E.; Ballvé, A.; Llauradó, A.; Gallardo, V.J.; Ariton, D.M.; Lallana, S.; López Maza, S.; Gadea, M.O.; Quibus, L.; Restrepo, J.L.; et al. Headache: A striking prodromal and persistent symptom, predictive of COVID-19 clinical evolution. Cephalalgia 2020, 13, 1410–1421. [Google Scholar] [CrossRef]
- Sia, J. Dizziness can be an early sole clinical manifestation for COVID-19 infection: A case report. J. Am. Coll. Emerg. Physicians Open 2020, 1, 1354–1356. [Google Scholar] [CrossRef] [PubMed]
- Spinato, G.; Fabbris, C.; Polesel, J.; Cazzador, D.; Borsetto, D.; Hopkins, C.; Boscolo-Rizzo, P. Alterations in Smell or Taste in Mildly Symptomatic Outpatients With SARS-CoV-2 Infection. JAMA 2020, 323, 2089–2090. [Google Scholar] [CrossRef] [Green Version]
- Kandemirli, S.G.; Altundag, A.; Yildirim, D.; Tekcan Sanli, D.E.; Saatci, O. Olfactory Bulb MRI and Paranasal Sinus CT Findings in Persistent COVID-19 Anosmia. Acad. Radiol. 2021, 28, 28–35. [Google Scholar] [CrossRef]
- Vargas-Gandica, J.; Winter, D.; Schnippe, R.; Rodriguez-Morales, A.G.; Mondragon, J.; Escalera-Antezana, J.P.; Trelles-Thorne, M.D.P.; Bonilla-Aldana, D.K.; Rodriguez-Morales, A.J.; Paniz-Mondolfi, A. Ageusia and anosmia, a common sign of COVID-19? A case series from four countries. J. Neurovirol. 2020, 26, 785–789. [Google Scholar] [CrossRef]
- Lechien, J.R.; Chiesa-Estomba, C.M.; De Siati, D.R.; Horoi, M.; Le Bon, S.D.; Rodriguez, A.; Dequanter, D.; Blecic, S.; El Afia, F.; Distinguin, L.; et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): A multicenter European study. Eur. Arch. Otorhinolaryngol. 2020, 277, 2251–2261. [Google Scholar] [CrossRef]
- Ebrahimpour, S.; Mohseni Afshar, Z.; Mohseni, S.; Masrour-Roudsari, J.; Oladzade, S.; Bayani, M.; Babazadeh, A. Neurologic manifestations in patients with COVID-19: A case report. Caspian J. Intern. Med. 2020, 11, 557–560. [Google Scholar] [CrossRef] [PubMed]
- Hampshire, A.; Trender, W.; Chamberlain, S.R.; Jolly, A.; Grant, J.E.; Patrick, F.; Mazibuko, N. Steve CR Williams, Joseph M Barnby, Peter Hellyer, Mitul A Mehta. Cognitive deficits in people who have recovered from COVID-19 relative to controls: An N = 84,285 online study. medRxiv 2020. [Google Scholar] [CrossRef]
- Guilmot, A.; Maldonado Slootjes, S.; Sellimi, A.; Bronchain, M.; Hanseeuw, B.; Belkhir, L.; Yombi, J.C.; De Greef, J.; Pothen, L.; Yildiz, H.; et al. Immune-mediated neurological syndromes in SARS-CoV-2-infected patients. J. Neurol. 2020, 30, 1–7. [Google Scholar] [CrossRef]
- Alkeridy, W.A.; Almaghlouth, I.; Alrashed, R.; Alayed, K.; Binkhamis, K.; Alsharidi, A.; Liu-Ambrose, T.A. Unique Presentation of Delirium in a Patient with Otherwise Asymptomatic COVID-19. J. Am. Geriatr. Soc. 2020, 68, 1382–1384. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo Zarazaga, A.; Delgado Parada, E.; Santamaría Núnez, M.; López Cruz, A.; Pardo Corral, M.; Ximénez-Carrillo, A. Hipótesis neuroinvasiva en un caso de delirium atípico en paciente con neumonía por COVID-19. Psiq. Biol. 2020. [Google Scholar] [CrossRef]
- Lu, S.; Wei, N.; Jiang, J.; Wu, L.; Sheng, J.; Zhou, J.; Fang, Q.; Chen, Y.; Zheng, S.; Chen, F.; et al. First report of manic-like symptoms in a COVID-19 patient with no previous history of a psychiatric disorder. J. Affect. Disord. 2020, 277, 337–340. [Google Scholar] [CrossRef] [PubMed]
- Méndez-Guerrero, A.; Laespada-García, M.I.; Gómez-Grande, A.; Ruiz-Ortiz, M.; Blanco-Palmero, V.A.; Azcarate-Diaz, F.J.; Rábano-Suárez, P.; Álvarez-Torres, E.; de Fuenmayor-Fernández de la Hoz, C.P.; Vega Pérez, D.; et al. Acute hypokinetic-rigid syndrome following SARS-CoV-2 infection. Neurology 2020, 95, e2109–e2118. [Google Scholar] [CrossRef]
- Panico, F.; Arini, A.; Cantone, P.; Crisci, C.; Trojano, L. Balint-Holmes syndrome due to stroke following SARS-CoV-2 infection: A single-case report. Neurol. Sci. 2020, 41, 3487–3489. [Google Scholar] [CrossRef]
- Elkhaled, W.; Ben Abid, F.; Akhtar, N.; Abukamar, M.R.; Ibrahim, W.H. A 23-Year-Old Man with SARS-CoV-2 Infection Who Presented with Auditory Hallucinations and Imaging Findings of Cytotoxic Lesions of the Corpus Callosum (CLOCC). Am. J. Case. Rep. 2020, 21, e928798. [Google Scholar] [CrossRef]
- Lin, J.; Lawson, E.C.; Verma, S.; Peterson, R.B.; Sidhu, R. Cytotoxic Lesion of the Corpus Callosum in an Adolescent with Multisystem Inflammatory Syndrome and SARS-CoV-2 Infection. AJNR Am. J. Neuroradiol. 2020, 41, 2017–2019. [Google Scholar] [CrossRef] [PubMed]
- Moreau, A.; Ego, A.; Vandergheynst, F.; Taccone, F.S.; Sadeghi, N.; Montesinos, I.; Gaspard, N.; Gorham, J. Cytotoxic lesions of the corpus callosum (CLOCCs) associated with SARS-CoV-2 infection. J. Neurol. 2020, 1–3. [Google Scholar] [CrossRef]
- Zanin, L.; Saraceno, G.; Panciani, P.P.; Renisi, G.; Signorini, L.; Migliorati, K.; Fontanella, M.M. SARS-CoV-2 can induce brain and spine demyelinating lesions. Acta Neurochir. 2020, 162, 1491–1494. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.M.; Cheng, V.C.; Hung, I.F.; Wong, M.M.; Chan, K.H.; Chan, K.S.; Kao, R.Y.; Poon, L.L.; Wong, C.L.; Guan, Y.; et al. Role of lopinavir/ritonavir in the treatment of SARS: Initial virological and clinical findings. Thorax 2004, 59, 252–256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thorlund, K.; Dron, L.; Park, J.; Hsu, G.; Forrest, J.I.; Mills, E. A real-time dashboard of clinical trials for COVID-19. Lancet. Digi. Health 2020, 2, e286–e287. [Google Scholar] [CrossRef]
- Savarino, A.; Boelaert, J.R.; Cassone, A.; Majori, G.; Cauda, R. Effects of chloroquine on viral infections: An old drug against today’s diseases? Lancet Infect. Dis. 2003, 3, 722–727. [Google Scholar] [CrossRef]
- Xu, X.; Han, M.; Li, T.; Sun, W.; Wang, D.; Fu, B.; Zhou, Y.; Zheng, X.; Yang, Y.; Li, X.; et al. Effective treatment of severe COVID-19 patients with tocilizumab. Proc. Natl. Acad. Sci. USA 2020, 117, 10970–10975. [Google Scholar] [CrossRef] [PubMed]
- Shang, C.; Zhuang, X.; Zhang, H.; Li, Y.; Zhu, Y.; Lu, J.; Ge, C.; Cong, J.; Li, T.; Tian, M.; et al. Inhibitors of endosomal acidification suppress SARS-CoV-2 replication and relieve viral pneumonia in hACE2 transgenic mice. Virol. J. 2021, 18, 46. [Google Scholar] [CrossRef]
- Diaz-Arocutipa, C.; Brañez-Condorena, A.; Hernandez, A.V. QTc prolongation in COVID-19 patients treated with hydroxychloroquine, chloroquine, azithromycin, or lopinavir/ritonavir: A systematic review and meta-analysis. Pharmacoepidemiol. Drug. Saf. 2021. [Google Scholar] [CrossRef]
- Richardson, P.; Griffin, I.; Tucker, C.; Smith, D.; Oechsle, O.; Phelan, A.; Rawling, M.; Savory, E.; Stebbing, J. Baricitinib as potential treatment for 2019-nCoV acute respiratory disease. Lancet 2020, 395, e30–e31. [Google Scholar] [CrossRef] [Green Version]
- Jaggers, G.K.; Watkins, B.A.; Rodriguez, R.L. COVID-19: Repositioning nutrition research for the next pandemic. Nutr. Res. 2020, 81, 1–6. [Google Scholar] [CrossRef]
- Klok, F.A.; Kruip, M.J.H.A.; van der Meer, N.J.M.; Arbous, M.S.; Gommers, D.A.M.P.J.; Kant., K.M.; Kaptein, F.H.J.; van Paassen, J.; Stals, M.A.M.; Huisman, M.V.; et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb. Res. 2020, 191, 145–147. [Google Scholar] [CrossRef] [PubMed]
- Barrett, C.D.; Moore, H.B.; Yaffe, M.B.; Moore, E.E. ISTH interim guidance on recognition and management of coagulopathy in COVID-19: A comment. J. Thromb. Haemost. 2020, 18, 2060–2063. [Google Scholar] [CrossRef] [PubMed]
- Moore, H.B.; Barrett, C.D.; Moore, E.E.; McIntyre, R.C.; Moore, P.K.; Talmor, D.S.; Moore, F.A.; Yaffe, M.B. Is there a role for tissue plasminogen activator as a novel treatment for refractory COVID-19 associated acute respiratory distress syndrome? J. Trauma Acute Care Surg. 2020, 88, 713–714. [Google Scholar] [CrossRef] [PubMed]
- Ong, W.Y.; Go, M.L.; Wang, D.Y.; Cheah, I.K.; Halliwell, B. Effects of Antimalarial Drugs on Neuroinflammation-Potential Use for Treatment of COVID-19-Related Neurologic Complications. Mol. Neurobiol. 2021, 58, 106–117. [Google Scholar] [CrossRef] [PubMed]
- Jaworowski, S.; Weiser, M.; Gropp, C.; Malka, M. Three Cases of COVID-19-related First Onset Brief Reactive Psychosis. Isr. Med. Assoc. J. 2020, 22, 612. [Google Scholar] [PubMed]
- Anmella, G.; Arbelo, N.; Fico, G.; Murru, A.; Llach, C.D.; Madero, S.; Gomes-da-Costa, S.; Imaz, M.L.; López-Pelayo, H.; Vieta, E.; et al. COVID-19 inpatients with psychiatric disorders: Real-world clinical recommendations from an expert team in consultation-liaison psychiatry. J. Affect. Disord. 2020, 274, 1062–1067. [Google Scholar] [CrossRef]
- Zeng, L.; Zhang, H.; He, Y.; Lai, B.; Huang, Z.; Lin, L.; Zhong, Z.; Guo, X. A severe COVID-19 case with schizophrenia as well as other chronic diseases. Braz. J. Med. Biol. Res. 2021, 54, e10426. [Google Scholar] [CrossRef] [PubMed]
- Castillo, R.R.; Quizon, G.R.A.; Juco, M.J.M.; Roman, A.D.E.; de Leon, D.G.; Punzalan, F.E.R.; Guingon, R.B.L.; Morales, D.D.; Tan, D.-X.; Reiter, R.J. Melatonin as adjuvant treatment for coronavirus disease 2019 pneumonia patients requiring hospitalization (MAC-19 PRO): A case series. Melatonin Res. 2020, 297–310. [Google Scholar] [CrossRef]
- Sher, Y.; Rabkin, B.; Maldonado, J.R.; Mohabir, P. COVID-19-Associated Hyperactive Intensive Care Unit Delirium with Proposed Pathophysiology and Treatment: A Case Report. Psychosomatics 2020, 61, 544–550. [Google Scholar] [CrossRef]
- Baller, E.B.; Hogan, C.S.; Fusunyan, M.A.; Ivkovic, A.; Luccarelli, J.W.; Madva, E.; Nisavic, M.; Praschan, N.; Quijije, N.V.; Beach, S.R.; et al. Neurocovid: Pharmacological Recommendations for Delirium Associated With COVID-19. Psychosomatics 2020, 61, 585–596. [Google Scholar] [CrossRef]
- Van Vuren, E.J.; Steyn, S.F.; Brink, C.B.; Möller, M.; Viljoen, F.P.; Harvey, B.H. The neuropsychiatric manifestations of COVID-19: Interactions with psychiatric illness and pharmacological treatment. Biomed. Pharmacother. 2021, 135, 111200. [Google Scholar] [CrossRef] [PubMed]
- El-Zein, R.S.; Cardinali, S.; Murphy, C.; Keeling, T. COVID-19-associated meningoencephalitis treated with intravenous immunoglobulin. BMJ Case Rep. 2020, 13, e237364. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B.; Gutteridge, J. Free Radical in Biology and Medicine, 5th ed.; Oxford University Press: New York, NY, USA, 2015. [Google Scholar] [CrossRef]
- Kim, H.J.; Kim, C.H.; Ryu, J.H.; Kim, M.J.; Park, C.Y.; Lee, J.M.; Holtzman, M.J.; Yoon, J.H. Reactive oxygen species induce antiviral innate immune response through IFN-λ regulation in human nasal epithelial cells. Am. J. Respir. Cell. Mol. Biol. 2013, 49, 855–865. [Google Scholar] [CrossRef]
- Khomich, O.A.; Kochetkov, S.N.; Bartosch, B.; Ivanov, A.V. Redox Biology of Respiratory Viral Infections. Viruses 2018, 10, 392. [Google Scholar] [CrossRef] [Green Version]
- Griffith, B.; Pendyala, S.; Hecker, L.; Lee, P.J.; Natarajan, V.; Thannickal, V.J. NOX enzymes and pulmonary disease. Antioxid. Redox. Signal. 2009, 11, 2505–2516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akki, R.; Fath, N.; Mohti, H. COVID-19: Oxidative Preconditioning as a Potential Therapeutic Approach. ACS Chem. Neurosci. 2020, 11, 3732–3740. [Google Scholar] [CrossRef]
- Komaravelli, N.; Casola, A. Respiratory Viral Infections and Subversion of Cellular Antioxidant Defenses. J. Pharmacogenom. Pharmacoproteom. 2014, 5, 1000141. [Google Scholar] [CrossRef]
- Laforge, M.; Elbim, C.; Frère, C.; Hémadi, M.; Massaad, C.; Nuss, P.; Benoliel, J.J.; Becker, C. Tissue damage from neutrophil-induced oxidative stress in COVID-19. Nat. Rev. Immunol. 2020, 20, 515–516. [Google Scholar] [CrossRef]
- Liu, Y.; Du, X.; Chen, J.; Jin, Y.; Peng, L.; Wang, H.; Luo, M.; Chen, L.; Zhao, Y. Neutrophil-to-lymphocyte ratio as an independent risk factor for mortality in hospitalized patients with COVID-19. J. Infect. 2020, 81, e6–e12. [Google Scholar] [CrossRef] [PubMed]
- Qin, C.; Zhou, L.; Hu, Z.; Zhang, S.; Yang, S.; Tao, Y.; Xie, C.; Ma, K.; Shang, K.; Wang, W.; et al. Dysregulation of Immune Response in Patients with Coronavirus 2019 (COVID-19) in Wuhan, China. Clin. Infect. Dis. 2020, 71, 762–768. [Google Scholar] [CrossRef]
- Wang, D.; Hu, B.; Hu, C.; Zhu, F.; Liu, X.; Zhang, J.; Wang, B.; Xiang, H.; Cheng, Z.; Xiong, Y.; et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA 2020, 323, 1061–1069. [Google Scholar] [CrossRef]
- Zhang, B.; Zhou, X.; Zhu, C.; Song, Y.; Feng, F.; Qiu, Y.; Feng, J.; Jia, Q.; Song, Q.; Zhu, B.; et al. Immune Phenotyping Based on the Neutrophil-to-Lymphocyte Ratio and IgG Level Predicts Disease Severity and Outcome for Patients With COVID-19. Front Mol. Biosci. 2020, 7, 157. [Google Scholar] [CrossRef] [PubMed]
- Schönrich, G.; Raftery, M.J.; Samstag, Y. Devilishly radical NETwork in COVID-19: Oxidative stress, neutrophil extracellular traps (NETs), and T cell suppression. Adv. Biol. Regul. 2020, 77, 100741. [Google Scholar] [CrossRef] [PubMed]
- Li, S.W.; Wang, C.Y.; Jou, Y.J.; Yang, T.C.; Huang, S.H.; Wan, L.; Lin, Y.J.; Lin, C.W. SARS coronavirus papain-like protease induces Egr-1-dependent up-regulation of TGF-β1 via ROS/p38 MAPK/STAT3 pathway. Sci. Rep. 2016, 6, 25754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Griendling, K.K.; Sorescu, D.; Lassègue, B.; Ushio-Fukai, M. Modulation of protein kinase activity and gene expression by reactive oxygen species and their role in vascular physiology and pathophysiology. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 2175–2183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dikalov, S. Cross talk between mitochondria and NADPH oxidases. Free Radic. Biol. Med. 2011, 51, 1289–1301. [Google Scholar] [CrossRef] [Green Version]
- Pignatelli, P.; Carnevale, R.; Cammisotto, V.; Lichtner, M.; Alessandri, F.; De Angelis, M.; Miele, M.C.; D’Ettorre, G.; Ruberto, F.; Venditti, M.; et al. Nox2 activation in Covid-19. Redox. Biol. 2020, 36, 101655. [Google Scholar] [CrossRef]
- Wen, H.; Gwathmey, J.K.; Xie, L.H. Oxidative stress-mediated effects of angiotensin II in the cardiovascular system. World J. Hypertens. 2012, 2, 34–44. [Google Scholar] [CrossRef]
- Wang, H.; Yang, P.; Liu, K.; Guo, F.; Zhang, Y.; Zhang, G.; Jiang, C. SARS coronavirus entry into host cells through a novel clathrin- and caveolae-independent endocytic pathway. Cell Res. 2008, 8, 290–301. [Google Scholar] [CrossRef] [Green Version]
- Beltrán-García, J.; Osca-Verdegal, R.; Pallardó, F.V.; Ferreres, J.; Rodríguez, M.; Mulet, S.; Sanchis-Gomar, F.; Carbonell, N.; García-Giménez, J.L. Oxidative Stress and Inflammation in COVID-19-Associated Sepsis: The Potential Role of Anti-Oxidant Therapy in Avoiding Disease Progression. Antioxidants 2020, 9, 936. [Google Scholar] [CrossRef] [PubMed]
- Suhail, S.; Zajac, J.; Fossum, C.; Lowater, H.; McCracken, C.; Severson, N.; Laatsch, B.; Narkiewicz-Jodko, A.; Johnson, B.; Liebau, J.; et al. Role of Oxidative Stress on SARS-CoV (SARS) and SARS-CoV-2 (COVID-19) Infection: A Review. Protein J. 2020, 39, 644–656. [Google Scholar] [CrossRef]
- Busse, L.W.; Chow, J.H.; McCurdy, M.T.; Khanna, A.K. COVID-19 and the RAAS-a potential role for angiotensin II? Crit. Care 2020, 24, 136. [Google Scholar] [CrossRef] [Green Version]
- Hati, S.; Bhattacharyya, S. Impact of Thiol-Disulfide Balance on the Binding of Covid-19 Spike Protein with Angiotensin-Converting Enzyme 2 Receptor. CS Omega 2020, 5, 16292–16298. [Google Scholar] [CrossRef]
- Rabelo, L.A.; Alenina, N.; Bader, M. ACE2-angiotensin-(1-7)-Mas axis and oxidative stress in cardiovascular disease. Hyper. Res. 2011, 34, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Blaser, H.; Dostert, C.; Mak, T.W.; Brenner, D. TNF and ROS Crosstalk in Inflammation. Trend. Cell. Biol. 2016, 26, 249–261. [Google Scholar] [CrossRef] [PubMed]
- Pennathur, S.; Heinecke, J.W. Oxidative stress and endothelial dysfunction in vascular disease. Curr. Diab. Rep. 2007, 4, 257–264. [Google Scholar] [CrossRef]
- Sandoval, R.; Lazcano, P.; Ferrari, F.; Pinto-Pardo, N.; González-Billault, C.; Utreras, E. TNF-α increases production of reactive oxygen species through Cdk5 activation in nociceptive neurons. Front. Physiol. 2018, 9, 65. [Google Scholar] [CrossRef] [Green Version]
- Cavezzi, A.; Troiani, E.; Corrao, S. COVID-19: Hemoglobin, iron, and hypoxia beyond inflammation. A narrative review. Clin. Pract. 2020, 10, 1271. [Google Scholar] [CrossRef] [PubMed]
- Saleh, J.; Peyssonnaux, C.; Singh, K.K.; Edeas, M. Mitochondria and microbiota dysfunction in COVID-19 pathogenesis. Mitochondrion 2020, 54, 1–7. [Google Scholar] [CrossRef]
- Jo, E.K.; Kim, J.K.; Shin, D.M.; Sasakawa, C. Molecular mechanisms regulating NLRP3 inflammasome activation. Cell. Mol. Immunol. 2016, 13, 148–159. [Google Scholar] [CrossRef] [Green Version]
- Naik, E.; Dixit, V.M. Mitochondrial reactive oxygen species drive proinflammatory cytokine production. J. Exp. Med. 2011, 208, 417–420. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wang, L.; Peng, L.; Chen, L.; Qin, Y.; Zhao, D.; Tan, S.; Yin, L.; Xu, J.; Zhou, C.; et al. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci. China Life Sci. 2020, 63, 364–374. [Google Scholar] [CrossRef] [Green Version]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Abdennour, L.; Zeghal, C.; Dème, M.; Puybasset, L. Interaction cerveau-poumon [Interaction brain-lungs]. Ann. Fr. Anesth. Reanim. 2012, 31, e101-7. [Google Scholar] [CrossRef]
- Olagnier, D.P. Identification of SARS-CoV2-mediated suppression of NRF2 signaling reveals a potent antiviral and anti-inflammatory activity of 4-octyl-itaconate and dimethyl fumarate. Nat. Commun. 2020, 11, 5419. [Google Scholar] [CrossRef]
- Cuadrado, A.; Pajares, M.; Benito, C.; Jiménez-Villegas, J.; Escoll, M.; Fernández-Ginés, R.; Garcia Yagüe, A.J.; Lastra, D.; Manda, G.; Rojo, A.I.; et al. Can Activation of NRF2 Be a Strategy against COVID-19? Trends. Pharmacol. Sci. 2020, 41, 598–610. [Google Scholar] [CrossRef]
- Cuadrado, A. Therapeutic targeting of the NRF2 and KEAP1 partnership in chronic diseases. Nat. Rev. Drug Discov. 2019, 18, 295–317. [Google Scholar] [CrossRef] [Green Version]
- Polonikov, A. Endogenous Deficiency of Glutathione as the Most Likely Cause of Serious Manifestations and Death in COVID-19 Patients. ACS Infect. Dis. 2020, 6, 1558–1562. [Google Scholar] [CrossRef]
- Abraham, E.C.; Taylor, J.F.; Lang, C.A. Influence of mouse age and erythrocyte age on glutathione metabolism. Biochem J. 1978, 174, 819–825. [Google Scholar] [CrossRef] [Green Version]
- Abouhashem, A.S.; Singh, K.; Azzazy, H.; Sen, C.K. Is Low Alveolar Type II Cell SOD3 in the Lungs of Elderly Linked to the Observed Severity of COVID-19? Antioxid. Redox. Signal. 2020, 33, 59–65. [Google Scholar] [CrossRef]
- Ling, C.Q. Traditional Chinese medicine is a resource for drug discovery against 2019 novel coronavirus (SARS-CoV-2). J. Integr. Med. 2020, 18, 87–88. [Google Scholar] [CrossRef] [PubMed]
- Mondal, S.; Karmakar, A.; Mallick, T.; Begum, N.A. Exploring the efficacy of naturally occurring biflavone based antioxidants towards the inhibition of the SARS-CoV-2 spike glycoprotein mediated membrane fusion. Virology 2021, 556, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Chikhale, R.V.; Gurav, S.S.; Patil, R.B.; Sinha, S.K.; Prasad, S.K.; Shakya, A.; Shrivastava, S.K.; Gurav, N.S.; Prasad, R.S. Sars-cov-2 host entry and replication inhibitors from Indian ginseng: An in-silico approach. J. Biomol. Struct. Dyn. 2020, 1–12. [Google Scholar] [CrossRef]
- El-Missiry, M.A.; Fekri, A.; Kesar, L.A.; Othman, A.I. Polyphenols are potential nutritional adjuvants for targeting COVID-19. Phytother. Res. 2020. [Google Scholar] [CrossRef]
- Su, H.; Yao, S.; Zhao, W.; Li, M.; Liu, J.; Shang, W.; Xie, H.; Ke, C.; Gao, M.; Yu, K.; et al. Discovery of baicalin and baicalein as novel, natural product inhibitors of SARS-CoV-2 3CL protease in vitro. BioRxiv 2020. [Google Scholar] [CrossRef] [Green Version]
- Da Silva Antonio, A.; Wiedemann, L.S.M.; Veiga-Junior, V.F. Natural products’ role against COVID-19. RSC Advan. 2020, 10, 23379–23393. [Google Scholar] [CrossRef]
- Bhardwaj, V.K.; Singh, R.; Sharma, J.; Rajendran, V.; Purohit, R.; Kumar, S. Identification of bioactive molecules from tea plant as SARS-CoV-2 main protease inhibitors. J. Biomol. Struct. Dyn. 2020, 1–10. [Google Scholar] [CrossRef]
- Huynh, T.; Wang, H.; Luan, B. In Silico Exploration of the Molecular Mechanism of Clinically Oriented Drugs for Possibly Inhibiting SARS-CoV-2’s Main Protease. J. Phys. Chem. Lett. 2020, 11, 4413–4420. [Google Scholar] [CrossRef]
- Enmozhi, S.K.; Raja, K.; Sebastine, I.; Joseph, J. Andrographolide as a potential inhibitor of SARS-CoV-2 main protease: An in silico approach. J. Biomol. Struct. Dyn. 2020, 1–10. [Google Scholar] [CrossRef]
- Gurung, A.B.; Ali, M.A.; Lee, J.; Farah, M.A.; Al-Anazi, K.M. Unravelling lead antiviral phytochemicals for the inhibition of SARS-CoV-2 Mpro enzyme through in silico approach. Life Sci. 2020, 255, 117831. [Google Scholar] [CrossRef]
- Caruso, F.; Singh, M.; Belli, S.; Berinato, M.; Rossi, M. Interrelated Mechanism by Which the Methide Quinone Celastrol, Obtained from the Roots of Tripterygium wilfordii, Inhibits Main Protease 3CLpro of COVID-19 and Acts as Superoxide Radical Scavenger. Int. J. Mol. Sci. 2020, 21, 9266. [Google Scholar] [CrossRef]
- Chidambaram, S.K.; Ali, D.; Alarifi, S.; Radhakrishnan, S.; Akbar, I. In silico molecular docking: Evaluation of coumarin based derivatives against SARS-CoV-2. J. Infect. Public Health 2020, 13, 1671–1677. [Google Scholar] [CrossRef]
- Feitosa, E.L.; Júnior, F.; Nery Neto, J.; Matos, L.; Moura, M.; Rosales, T.O.; De Freitas, G. COVID-19: Rational discovery of the therapeutic potential of Melatonin as a SARS-CoV-2 main Protease Inhibitor. Int. J. Med. Sci. 2020, 17, 2133–2146. [Google Scholar] [CrossRef]
- Liang, J.; Karagiannis, C.; Pitsillou, E.; Darmawan, K.K.; Ng, K.; Hung, A.; Karagiannis, T.C. Site mapping and small molecule blind docking reveal a possible target site on the SARS-CoV-2 main protease dimer interface. Comput. Biol. Chem. 2020, 89, 107372. [Google Scholar] [CrossRef]
- Kodchakorn, K.; Poovorawan, Y.; Suwannakarn, K.; Kongtawelert, P. Molecular modelling investigation for drugs and nutraceuticals against protease of SARS-CoV-2. J. Mol. Graph. Model. 2020, 101, 107717. [Google Scholar] [CrossRef]
- Pandey, A.K.; Verma, S. An in-silico evaluation of dietary components for structural inhibition of SARS-Cov-2 main protease. J. Biomol. Struct. Dyn. 2020, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Abian, O.; Ortega-Alarcon, D.; Jimenez-Alesanco, A.; Ceballos-Laita, L.; Vega, S.; Reyburn, H.T.; Rizzuti, B.; Velazquez-Campoy, A. Structural stability of SARS-CoV-2 3CLpro and identification of quercetin as an inhibitor by experimental screening. Int. J. Biol. Macromol. 2020, 164, 1693–1703. [Google Scholar] [CrossRef]
- Matondo, A.; Kilembe, J.T.; Mwanangombo, D.T.; Nsimba, B.M.; Gbolo, B.Z.; Bongo, G.N.; Ngbolua, K.-t.-N.; Tshilanda, D.D.; Tshibangu, D.S.T.; Mudogo, V.; et al. Facing COVID-19 via anti-inammatory mechanism of action: Molecular docking and Pharmacokinetic studies of six anti-inammatory compounds derived from Passiora edulis. Res. Square 2020. [Google Scholar] [CrossRef]
- Mosquera-Yuqui, F.; Lopez-Guerra, N.; Moncayo-Palacio, E.A. Targeting the 3CLpro and RdRp of SARS-CoV-2 with phytochemicals from medicinal plants of the Andean Region: Molecular docking and molecular dynamics simulations. J. Biomol. Struct. Dyn. 2020, 1, 1–14. [Google Scholar] [CrossRef]
- Lung, J.; Lin, Y.S.; Yang, Y.H.; Chou, Y.L.; Shu, L.H.; Cheng, Y.C.; Liu, H.T.; Wu, C.Y. The potential chemical structure of anti-SARS-CoV-2 RNA-dependent RNA polymerase. J. Med. Virol. 2020, 92, 693–697. [Google Scholar] [CrossRef] [PubMed]
- Kandeel, M.; Kitade, Y.; Almubarak, A. Repurposing FDA-approved phytomedicines, natural products, antivirals and cell protectives against SARS-CoV-2 (COVID-19) RNA-dependent RNA polymerase. PeerJ 2020, 8, e10480. [Google Scholar] [CrossRef]
- Mhatre, S.; Naik, S.; Patravale, V. A molecular docking study of EGCG and theaflavin digallate with the druggable targets of SARS-CoV-2. Comput. Biol. Med. 2021, 129, 104137. [Google Scholar] [CrossRef] [PubMed]
- Rahman, F.; Tabrez, S.; Ali, R.; Alqahtani, A.S.; Ahmed, M.Z.; Rub, A. Molecular docking analysis of rutin reveals possible inhibition of SARS-CoV-2 vital proteins. J. Tradit. Complement. Med. 2021, 11, 173–179. [Google Scholar] [CrossRef]
- Utomo, R.Y.; Ikawati, M.; Meiyanto, E. Revealing the Potency of Citrus and Galangal Constituents to Halt SARS-CoV-2 Infection. Preprints 2020. [Google Scholar] [CrossRef] [Green Version]
- Chikhale, R.V.; Sinha, S.K.; Patil, R.B.; Prasad, S.K.; Shakya, A.; Gurav, N.; Prasad, R.; Dhaswadikar, S.R.; Wanjari, M.; Gurav, S.S. In-silico investigation of phytochemicals from Asparagus racemosus as plausible antiviral agent in COVID-19. J. Biomol. Struct. Dyn. 2020, 1–15. [Google Scholar] [CrossRef]
- Jayawardena, R.; Sooriyaarachchi, P.; Chourdakis, M.; Jeewandara, C.; Ranasinghe, P. Enhancing immunity in viral infections, with special emphasis on COVID-19: A review. Diabetes Metab. Syndr. 2020, 14, 367–382. [Google Scholar] [CrossRef]
- Reddy, G.J.; Hema, K.; Dodoala, S.; Koganti, B. Unravelling high-affinity binding compounds towards transmembrane protease serine 2 enzyme in treating SARS-CoV-2 infection using molecular modelling and docking studies. Eur. J. Pharmacol. 2021, 890, 173688. [Google Scholar] [CrossRef]
- Kumar, S.; Kashyap, P.; Chowdhury, S.; Kumar, S.; Panwar, A.; Kumar, A. Identification of phytochemicals as potential therapeutic agents that binds to Nsp15 protein target of coronavirus (SARS-CoV-2) that are capable of inhibiting virus replication. Phytomedicine 2020, 153317. [Google Scholar] [CrossRef] [PubMed]
- Maiti, S.; Banerjee, A.; Nazmeen, A.; Kanwar, M.; Das, S. Active-site Molecular docking of Nigellidine with nucleocapsid- NSP2-MPro of COVID-19 and to human IL1R-IL6R and strong antioxidant role of Nigella-sativa in experimental rats. J. Drug. Target. 2020, 2, 1–23. [Google Scholar] [CrossRef]
- Rolta, R.; Yadav, R.; Salaria, D.; Trivedi, S.; Imran, M.; Sourirajan, A.; Baumler, D.J.; Dev, K. In silico screening of hundred phytocompounds of ten medicinal plants as potential inhibitors of nucleocapsid phosphoprotein of COVID-19: an approach to prevent virus assembly. J. Biomol. Struct. Dyn. 2020, 1–8. [Google Scholar] [CrossRef]
- Bhowmik, D.; Nandi, R.; Jagadeesan, R.; Kumar, N.; Prakash, A.; Kumar, D. Identification of potential inhibitors against SARS-CoV-2 by targeting proteins responsible for envelope formation and virion assembly using docking based virtual screening, and pharmacokinetics approaches. Infect. Genet. Evol. 2020, 84, 104451. [Google Scholar] [CrossRef]
- Verma, V.A.; Saundane, A.R.; Meti, R.S.; Vennapu, D.R. Synthesis of novel indolo[3,2-c]isoquinoline derivatives bearing pyrimidine, piperazine rings and their biological evaluation and docking studies against COVID-19 virus main protease. J. Mol. Struct. 2021, 1229, 129829. [Google Scholar] [CrossRef]
- Sepay, N.; Sekar, A.; Halder, U.C.; Alarifi, A.; Afzal, M. Anti-COVID-19 terpenoid from marine sources: A docking, admet and molecular dynamics study. J. Mol. Struct. 2021, 1228, 129433. [Google Scholar] [CrossRef] [PubMed]
- Alaşalvar, C.; Öztürk, N.; Gökce, H.; Güder, A.; Menteşe, E.; Bektaş, H. Synthesis, structural, spectral, antioxidant, bioactivity and molecular docking investigations of a novel triazole derivative. J. Biomol. Struct. Dyn. 2021, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Ranjbar, A.; Jamshidi, M.; Torabi, S. Molecular modelling of the antiviral action of Resveratrol derivatives against the activity of two novel SARS CoV-2 and 2019-nCoV receptors. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 7834–7844. [Google Scholar] [CrossRef] [PubMed]
- Marinella, M.A. Indomethacin and resveratrol as potential treatment adjuncts for SARS-CoV-2/COVID-19. Int. J. Clin. Pract. 2020, 74, e13535. [Google Scholar] [CrossRef] [PubMed]
- Zahedipour, F.; Hosseini, S.A.; Sathyapalan, T.; Majeed, M.; Jamialahmadi, T.; Al-Rasadi, K.; Banach, M.; Sahebkar, A. Potential effects of curcumin in the treatment of COVID-19 infection. Phytother. Res. 2020, 34, 2911–2920. [Google Scholar] [CrossRef]
- Zhou, N.; Yang, X.; Huang, A.; Chen, Z. The potential mechanism of N-acetylcysteine in treating COVID-19. Curr. Pharm. Biotechnol. 2020. [Google Scholar] [CrossRef]
- Paraiso, I.L.; Revel, J.S.; Stevens, J.F. Potential use of polyphenols in the battle against COVID-19. Curr. Opin. Food. Sci. 2020, 32, 149–155. [Google Scholar] [CrossRef]
- Ahmad, S.; Abbasi, H.W.; Shahid, S.; Gul, S.; Abbasi, S.W. Molecular docking, simulation and MM-PBSA studies of Nigella sativa compounds: A computational quest to identify potential natural antiviral for COVID-19 treatment. J. Biomol. Struct. Dyn. 2020, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ohta, S. Direct targets and subsequent pathways for molecular hydrogen to exert multiple functions: Focusing on interventions in radical reactions. Curr. Pharm. Des. 2021, 27, 595–609. [Google Scholar] [CrossRef]
- Jorge-Aarón, R.M.; Rosa-Ester, M.P. N-acetylcysteine as a potential treatment for COVID-19. Future Microbiol. 2020, 15, 959–962. [Google Scholar] [CrossRef]
- Banerjee, A.; Czinn, S.J.; Reiter, R.J.; Blanchard, T.G. Crosstalk between endoplasmic reticulum stress and anti-viral activities: A novel therapeutic target for COVID-19. Life Sci. 2020, 255, 117842. [Google Scholar] [CrossRef]
- McCord, J.M.; Hybertson, B.M.; Cota-Gomez, A.; Geraci, K.P.; Gao, B. Nrf2 Activator PB125® as a Potential Therapeutic Agent against COVID-19. Antioxidants 2020, 9, 518. [Google Scholar] [CrossRef]
- Haggag, Y.A.; El-Ashmawy, N.E.; Okasha, K.M. Is hesperidin essential for prophylaxis and treatment of COVID-19 Infection? Med. Hypotheses 2020, 44, 109957. [Google Scholar] [CrossRef]
- Takeda, Y.; Jamsransuren, D.; Matsuda, S.; Crea, R.; Ogawa, H. The SARS-CoV-2-Inactivating Activity of Hydroxytyrosol-Rich Aqueous Olive Pulp Extract (HIDROX®) and Its Use as a Virucidal Cream for Topical Application. Viruses 2021, 13, 232. [Google Scholar] [CrossRef] [PubMed]
- Mattioli, A.V.; Toni, S.; Coppi, F.; Farinetti, A. Practical tips for prevention of cardiovascular disease in women after quarantine for COVID-19 disease. Acta Biomed. 2020, 91, e2020127. [Google Scholar] [CrossRef]
- Becker, R.C. Covid-19 treatment update: Follow the scientific evidence. J. Thromb. Thrombolysis. 2020, 50, 43–53. [Google Scholar] [CrossRef] [Green Version]
- Panyod, S.; Ho, C.T.; Sheen, L.Y. Dietary therapy and herbal medicine for COVID-19 prevention: A review and perspective. J. Tradit. Complement. Med. 2020, 10, 420–427. [Google Scholar] [CrossRef]
- Zhang, D.; Guo, R.; Lei, L.; Liu, H.; Wang, Y.; Wang, Y.; Dai, T.; Zhang, T.; Lai, Y.; Wang, J.; et al. Frontline Science: COVID-19 infection induces readily detectable morphologic and inflammation-related phenotypic changes in peripheral blood monocytes. J. Leukoc. Biol. 2021, 109, 13–22. [Google Scholar] [CrossRef]
- Messina, G.; Polito, R.; Monda, V.; Cipolloni, L.; Di Nunno, N.; Di Mizio, G.; Murabito, P.; Carotenuto, M.; Messina, A.; Pisanelli, D.; et al. Functional Role of Dietary Intervention to Improve the Outcome of COVID-19: A Hypothesis of Work. Int. J. Mol. Sci. 2020, 21, 3104. [Google Scholar] [CrossRef]
- Grant, W.B.; Lahore, H.; McDonnell, S.L.; Baggerly, C.A.; French, C.B.; Aliano, J.L.; Bhattoa, H.P. Evidence that vitamin D supplementation could reduce risk of influenza and COVID-19 infections and deaths. Nutrients 2020, 12, 988. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Annweiler, C.; Beaudenon, M.; Gautier, J.; Simon, R.; Dubée, V.; Gonsard, J.; Parot-Schinkel, E.; COVIT-TRIAL Study Group. COvid-19 and high-dose VITamin D supplementation TRIAL in high-risk older patients (COVIT-TRIAL): Study protocol for a randomized controlled trial. Trials 2020, 21, 1031. [Google Scholar] [CrossRef] [PubMed]
- De Las Heras, N.; Martín Giménez, V.M.; Ferder, L.; Manucha, W.; Lahera, V. Implications of Oxidative Stress and Potential Role of Mitochondrial Dysfunction in COVID-19: Therapeutic Effects of Vitamin D. Antioxidants 2020, 9, 897. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.; Xu, M.; Wang, G.; Lv, J.; Ma, X.; Guo, Y.; Zhang, D.; Yang, H.; Jiang, W.; Deng, F.; et al. The efficiency and safety of high-dose vitamin C in patients with COVID-19: A retrospective cohort study. Aging 2021, 13, 7020–7034. [Google Scholar] [CrossRef] [PubMed]
- Zh Zhao, B.; Ling, Y.; Li, J.; Peng, Y.; Huang, J.; Wang, Y.; Qu, H.; Gao, Y.; Li, Y.; Hu, B.; et al. Beneficial aspects of high dose intravenous vitamin C on patients with COVID-19 pneumonia in severe condition: A retrospective case series study. Ann. Palliat. Med. 2021, 10, 1599–1609. [Google Scholar] [CrossRef]
- Oyagbemi, A.A.; Ajibade, T.O.; Aboua, Y.G.; Gbadamosi, I.T.; Adedapo, A.; Aro, A.O.; Adejumobi, O.A.; Thamahane-Katengua, E.; Omobowale, T.O.; Falayi, O.O.; et al. Potential health benefits of zinc supplementation for the management of COVID-19 pandemic. J. Food Biochem. 2021, 45, e13604. [Google Scholar] [CrossRef]
- Keflie, T.S.; Biesalski, H.K. Micronutrients and bioactive substances: Their potential roles in combating COVID-19. Nutrition 2020, 84, 111103. [Google Scholar] [CrossRef]
- Wongchitrat, P.; Shukla, M.; Sharma, R.; Govitrapong, P.; Reiter, R.J. Role of Melatonin on Virus-Induced Neuropathogenesis-A Concomitant Therapeutic Strategy to Understand SARS-CoV-2 Infection. Antioxidants 2021, 10, 47. [Google Scholar] [CrossRef]
- Babaei, F.; Nassiri-Asl, M.; Hosseinzadeh, H. Curcumin (a constituent of turmeric): New treatment option against COVID-19. Food. Sci. Nutr. 2020, 8, 5215–5227. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, M.; Luo, G.; Qian, X.; Wu, C.; Zhang, Y.; Chen, B.; Leung, E.L.; Tang, Y. Experience of N-acetylcysteine airway management in the successful treatment of one case of critical condition with COVID-19: A case report. Medicine 2020, 99, e22577. [Google Scholar] [CrossRef] [PubMed]
- Carothers, C.; Birrer, K.; Vo, M. Acetylcysteine for the Treatment of Suspected Remdesivir-Associated Acute Liver Failure in COVID-19: A Case Series. Pharmacotherapy 2020, 40, 1166–1171. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, J.O.G.; Jynge, P.; Ignarro, L.J. May Mangafodipir or Other SOD Mimetics Contribute to Better Care in COVID-19 Patients? Antioxidants 2020, 9, 971. [Google Scholar] [CrossRef]
- Dalili, N.; Kashefizadeh, A.; Nafar, M.; Poorrezagholi, F.; Firouzan, A.; Samadian, F.; Samavat, S.; Ziaie, S.; Fatemizadeh, S. Adding Colchicine to the Antiretroviral Medication—Lopinavir/Ritonavir (Kaletra) in Hospitalized Patients with Non-Severe Covid-19 Pneumonia: A Structured Summary of a Study Protocol for a Randomized Controlled Trial. Trials 2020, 21, 489. [Google Scholar] [CrossRef] [PubMed]
- Zhong, M.; Sun, A.; Xiao, T.; Yao, G.; Sang, L.; Zheng, L.; Zhang, L.; Jin, X.; Xu, W.; Yang, W.; et al. A Randomized, Single-blind, Group sequential, Active-controlled Study to evaluate the clinical efficacy and safety of α-Lipoic acid for critically ill patients with coronavirus disease 2019(COVID-19). MedRxiv 2020. [Google Scholar] [CrossRef] [Green Version]
- Horowitz, R.I.; Freeman, P.R.; Bruzzese, J. Efficacy of glutathione therapy in relieving dyspnea associated with COVID-19 pneumonia: A report of 2 cases. Respir. Med. Case Rep. 2020, 30, 101063. [Google Scholar] [CrossRef] [PubMed]
- Bousquet, J.; Le Moing, V.; Blain, H.; Czarlewski, W.; Zuberbier, T.; de la Torre, R.; Pizarro Lozano, N.; Reynes, J.; Bedbrook, A.; Cristol, J.P.; et al. Efficacy of broccoli and glucoraphanin in COVID-19: From hypothesis to proof-of-concept with three experimental clinical cases. World Allergy Organ. J. 2020, 14, 100498. [Google Scholar] [CrossRef]
- Aliter, K.F.; Al-Horani, R.A. Potential Therapeutic Benefits of Dipyridamole in COVID-19 Patients. Curr. Pharm. Des. 2020, 27, 866–875. [Google Scholar] [CrossRef]
- Malinowska, B.; Baranowska-Kuczko, M.; Kicman, A.; Schlicker, E. Opportunities, Challenges and Pitfalls of Using Cannabidiol as an Adjuvant Drug in COVID-19. Int. J. Mol. Sci. 2021, 22, 1986. [Google Scholar] [CrossRef]
- Tan, C.W.; Ho, L.P.; Kalimuddin, S.; Cherng, B.; Teh, Y.E.; Thien, S.Y.; Wong, H.M.; Tern, P.; Chandran, M.; Chay, J.; et al. Cohort study to evaluate the effect of vitamin D, magnesium, and vitamin B12 in combination on progression to severe outcomes in older patients with coronavirus (COVID-19). Nutrition 2020, 79–80, 111017. [Google Scholar] [CrossRef]
- Ding, H.; Deng, W.; Ding, L.; Ye, X.; Yin, S.; Huang, W. Glycyrrhetinic acid and its derivatives as potential alternative medicine to relieve symptoms in nonhospitalized COVID-19 patients. J. Med. Virol. 2020, 92, 2200–2204. [Google Scholar] [CrossRef]
- Cardinali, D.P.; Brown, G.M.; Pandi-Perumal, S.R. Can Melatonin Be a Potential “Silver Bullet” in Treating COVID-19 Patients? Diseases 2020, 8, 44. [Google Scholar] [CrossRef] [PubMed]
- Limanaqi, F.; Busceti, C.L.; Biagioni, F.; Lazzeri, G.; Forte, M.; Schiavon, S.; Sciarretta, S.; Frati, G.; Fornai, F.F. Cell Clearing Systems as Targets of Polyphenols in Viral Infections: Potential Implications for COVID-19 Pathogenesis. Antioxidants 2020, 9, 1105. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.; Min, D.S.; Park, H.; Kim, H.P. Flavonoids interfere with NLRP3 inflammasome activation. Toxicol. Appl. Pharmacol. 2018, 355, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Barré, J.; Sabatier, J.M.; Annweiler, C. Montelukast Drug May Improve COVID-19 Prognosis: A Review of Evidence. Front. Pharmacol. 2020, 11, 1344. [Google Scholar] [CrossRef] [PubMed]
- Lupon, E.; Lellouch, A.G.; Zal, F.; Cetrulo, C.L.; Lantieri, L.A. Combating hypoxemia in COVID-19 patients with a natural oxygen carrier, HEMO2Life® (M101). Med. Hypotheses 2021, 146, 110421. [Google Scholar] [CrossRef] [PubMed]
- Maurya, V.K.; Kumar, S.; Prasad, A.K.; Bhatt, M.L.B.; Saxena, S.K. Structure-based drug designing for potential antiviral activity of selected natural products from Ayurveda against SARS-CoV-2 spike glycoprotein and its cellular receptor. Virusdisease 2020, 31, 179–193. [Google Scholar] [CrossRef] [PubMed]
- Koh, S.S.; Ooi, S.C.; Lui, N.M.; Qiong, C.; Ho, L.T.; Cheah, I.K.; Halliwell, B.; Herr, D.R.; Ong, W.Y. Effect of Ergothioneine on 7-Ketocholesterol-Induced Endothelial Injury. Neuromolecular. Med. 2020, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Cheah, I.K.; Halliwell, B. Could Ergothioneine Aid in the Treatment of Coronavirus Patients? Antioxidants 2020, 9, 595. [Google Scholar] [CrossRef]
- Yao, Z.H.; Yao, X.L.; Zhang, Y.; Zhang, S.F.; Hu, J.C. Luteolin Could Improve Cognitive Dysfunction by Inhibiting Neuroinflammation. Neurochem. Res. 2018, 43, 806–820. [Google Scholar] [CrossRef] [PubMed]
- Kempuraj, D.; Thangavel, R.; Kempuraj, D.D.; Ahmed, M.E.; Selvakumar, G.P.; Raikwar, S.P.; Zaheer, S.A.; Iyer, S.S.; Govindarajan, R.; Chandrasekaran, P.N.; et al. Neuroprotective effects of flavone luteolin in neuroinflammation and neurotrauma. Biofactors 2021, 47, 190–197. [Google Scholar] [CrossRef]
- Kempuraj, D.; Selvakumar, G.P.; Ahmed, M.E.; Raikwar, S.P.; Thangavel, R.; Khan, A.; Zaheer, S.A.; Iyer, S.S.; Burton, C.; James, D.; et al. COVID-19, Mast Cells, Cytokine Storm, Psychological Stress, and Neuroinflammation. Neuroscientist 2020, 26, 402–414. [Google Scholar] [CrossRef]
- Ribeiro, D.E.; Oliveira-Giacomelli, Á.; Glaser, T.; Arnaud-Sampaio, V.F.; Andrejew, R.; Dieckmann, L.; Baranova, J.; Lameu, C.; Ratajczak, M.Z.; Ulrich, H. Hyperactivation of P2X7 receptors as a culprit of COVID-19 neuropathology. Mol. Psychiatry 2020, 1–16. [Google Scholar] [CrossRef]
- Uckun, F.M.; Carlson, J.; Orhan, C.; Powell, J.; Pizzimenti, N.M.; van Wyk, H.; Ozercan, I.H.; Volk, M.; Sahin, K. Rejuveinix Shows a Favorable Clinical Safety Profile in Human Subjects and Exhibits Potent Preclinical Protective Activity in the Lipopolysaccharide-Galactosamine Mouse Model of Acute Respiratory Distress Syndrome and Multi-Organ Failure. Front. Pharmacol. 2020, 11, 594321. [Google Scholar] [CrossRef]
- Lopachev, A.V.; Kazanskaya, R.B.; Khutorova, A.V.; Fedorova, T.N. An overview of the pathogenic mechanisms involved in severe cases of COVID-19 infection, and the proposal of salicyl-carnosine as a potential drug for its treatment. Eur. J. Pharmacol. 2020, 886, 173457. [Google Scholar] [CrossRef]
- Nagoor Meeran, M.F.; Sharma, C.; Goyal, S.N.; Kumar, S.; Ojha, S. CB2 receptor-selective agonists as candidates for targeting infection, inflammation, and immunity in SARS-CoV-2 infections. Drug Dev. Res. 2021, 82, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Berretta, A.A.; Silveira, M.A.D.; Cóndor Capcha, J.M.; De Jong, D. Propolis and its potential against SARS-CoV-2 infection mechanisms and COVID-19 disease: Running title: Propolis against SARS-CoV-2 infection and COVID-19. Biomed. Pharmacother. 2020, 131, 110622. [Google Scholar] [CrossRef] [PubMed]
- Kulyar, M.F.; Li, R.; Mehmood, K.; Waqas, M.; Li, K.; Li, J. Potential influence of Nagella sativa (Black cumin) in reinforcing immune system: A hope to decelerate the COVID-19 pandemic. Phytomedicine 2020, 85, 153277. [Google Scholar] [CrossRef]
- Savran, M.; Aslankoc, R.; Ozmen, O.; Erzurumlu, Y.; Savas, H.B.; Temel, E.N.; Kosar, P.A.; Boztepe, S. Agomelatine could prevent brain and cerebellum injury against LPS-induced neuroinflammation in rats. Cytokine 2020, 127, 154957. [Google Scholar] [CrossRef] [PubMed]
- Hernández, A.; Papadakos, P.J.; Torres, A.; González, D.A.; Vives, M.; Ferrando, C.; Baeza, J. Two known therapies could be useful as adjuvant therapy in critical patients infected by COVID-19. Rev. Esp. Anestesiol. Reanim. 2020, 67, 245–252. [Google Scholar] [CrossRef] [PubMed]
- U.S. National Library of Medicine. ClinicalTrials Page. Available online: www.clinicaltrials.gov (accessed on 22 April 2021).


| Type | Neurological Complications after COVID-19 Infection | Patients’ Origin | References |
|---|---|---|---|
| Inflammatory | Encephalitis Meningoencephalitis Cord myelopathy encephalitis Hypoxic encephalitis Autoinmune meningoencephalitis Acute-disseminated encephalomyelitis Autoimmune encephalitis Diffuse post hypoxic leukoencephalopathy Acute necrotizing encephalopathy Guillain–Barré Syndrome Guillain–Barré Syndrome associated with a cerebral vasculitis-like pattern Cerebillitis Mixed inflammatory cell Posterior reversible encephalopathy syndrome | Italy Iran United States Brazil United Kingdom India Egypt Mexico Canada Spain South Africa Netherlands Belgium France Peru Japan Germany Sweden | [62,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108] |
| Vascular | Hemorrhage (intracerebral, subarachnoid, and intracranial) Multi-territory hemorrhagic infarctions Microbleeds masquerades Cerebral venous sinus thrombosis Embolic stroke in the right insula and left cerebellum Microinfarcts throughout the cortex Posterior cerebral artery infarct Middle cerebral artery territory infarcts Cuffing of intracerebral blood vessels distant from the infarcts Left cerebral small subdural hematoma with mild brain edema Vasculitis Perfusion abnormalities in brain Large vessel stroke Small subcortical infarcts Brain microvascular occlusive disorder Secondary acute ischemic stroke | United States South Africa Switzerland Germany Mexico India Saudi Arabia Brazil Japan Italy Spain China Turkey | [95,97,106,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126] |
| Sensorial | Headache Vertigo Anosmia Ageusia Altered taste Migraine-like features Vision impairment Dizziness | Spain India Egypt China Canada Italy Turkey Germany United States Venezuela Bolivia | [72,97,98,127,128,129,130,131,132] |
| Behavioral | Confusion Seizure Convulsions Cognitive decay coma Neuropsychiatric disorder Delirium Maniac-like symptoms Depression Altered mental status Psychosis Dementia-like syndrome Dysexecutive syndrome | France China Iran Egypt Saudi Arabia Belgium Spain India United Kingdom | [69,72,96,97,98,125,133,134,135,136,137,138] |
| Peripheral | Peripheral neuropathy Myasthenia gravis Symmetric hypokinetic-rigid syndrome Cranial neuropathy Nerve pain Bell’s palsy Balint–Holmes’ syndrome Ataxia Anti-diuretic hormone secretion | Belgium Egypt Spain China India Italy | [72,97,98,99,135,139,140] |
| Anatomical lesions | Transtentorial herniation Cytotoxic lesions of the corpus callosum Diffuse corticospinal tract Brain and spine demyelinating lesions Pneumocephalus | United States Italy Saudi Arabia France | [69,125,126,141,142,143,144] |
| Type | Neurological Condition | Treatment Protocol | Reference |
|---|---|---|---|
| Immunological |
|
| [83] |
| [99] | ||
|
| [62,101] | |
|
| [164] | |
|
| [92] | |
| [93] | ||
|
| [100] | |
|
| [86] | |
|
| [94] | |
|
| [108] | |
|
| [144] | |
| Anatomical |
|
| [143] |
| Behavioral or Psychiatric |
|
| [99] |
|
| [136] | |
|
| [139] | |
| Vascular |
|
| [110] |
|
| [116] | |
|
| [120] | |
|
| [113] | |
|
| [125] |
| Antioxidant(s) | Protocol | Neurological or Psychiatric Conditions Assessed |
|---|---|---|
| Vitamin C |
|
|
| Zinc and vitamin C |
|
|
|
| |
|
| |
|
| |
| Hydroxychloroquine, vitamin C, vitamin D, and zinc |
|
|
| Melatonin |
|
|
| Glycine and N-acetylcysteine |
|
|
| Glycine |
|
|
| Vitamin C, vitamin E, melatonin, and N-acetylcysteine |
|
|
| Previfenon® |
|
|
| Hesperidin and diosmin |
|
|
| Fuzheng Huayu Tablet (FZHY) and vitamin C |
|
|
| Resveratrol |
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cárdenas-Rodríguez, N.; Bandala, C.; Vanoye-Carlo, A.; Ignacio-Mejía, I.; Gómez-Manzo, S.; Hernández-Cruz, E.Y.; Pedraza-Chaverri, J.; Carmona-Aparicio, L.; Hernández-Ochoa, B. Use of Antioxidants for the Neuro-Therapeutic Management of COVID-19. Antioxidants 2021, 10, 971. https://doi.org/10.3390/antiox10060971
Cárdenas-Rodríguez N, Bandala C, Vanoye-Carlo A, Ignacio-Mejía I, Gómez-Manzo S, Hernández-Cruz EY, Pedraza-Chaverri J, Carmona-Aparicio L, Hernández-Ochoa B. Use of Antioxidants for the Neuro-Therapeutic Management of COVID-19. Antioxidants. 2021; 10(6):971. https://doi.org/10.3390/antiox10060971
Chicago/Turabian StyleCárdenas-Rodríguez, Noemí, Cindy Bandala, América Vanoye-Carlo, Iván Ignacio-Mejía, Saúl Gómez-Manzo, Estefani Yaquelin Hernández-Cruz, José Pedraza-Chaverri, Liliana Carmona-Aparicio, and Beatriz Hernández-Ochoa. 2021. "Use of Antioxidants for the Neuro-Therapeutic Management of COVID-19" Antioxidants 10, no. 6: 971. https://doi.org/10.3390/antiox10060971