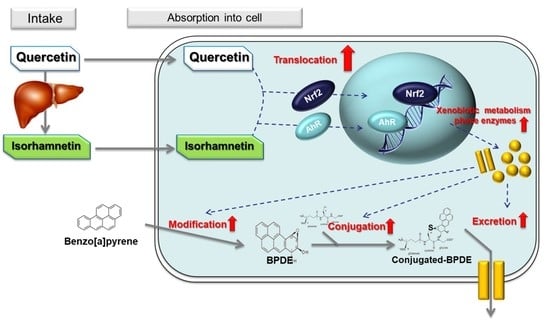
Quercetin and Isorhamnetin Attenuate Benzo[a]pyrene-Induced Toxicity by Modulating Detoxification Enzymes through the AhR and NRF2 Signaling Pathways
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Animals and Housing
2.3. Cell Culture and Treatment
2.4. Cell Viability Analysis
2.5. Quantification of 8-Hydroxydeoxyguanosine (8-oxo-dG)
2.6. BPDE-DNA Adduct Formation Analysis
2.7. Intracellular Metabolite Extraction and HPLC Analysis
2.8. Quantitative Reverse Transcription-Polymerase Chain Reaction (qRT-PCR)
2.9. Western Blot Analysis
2.10. Immunofluorescence Analysis
2.11. Statistical Analysis
3. Results
3.1. Cytoprotective Effect of Quercetin and Its Metabolites Against B[A]P-Induced Toxicity
3.2. Anti-genotoxic Effects Of Quercetin and Isorhamnetin via The Reduction of Intracellular B[A]P and Metabolites
3.3. Effects of Quercetin and Isorhamnetin on Phase Enzyme Expression
3.4. Effect of Quercetin and Isorhamnetin on Ahr and NRF2 Translocation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lee, T.; Puligundla, P.; Mok, C. Degradation of benzo[a]pyrene on glass slides and in food samples by low-pressure cold plasma. Food Chem. 2019, 286, 624–628. [Google Scholar] [CrossRef]
- Aygun, S.F.; Kabadayi, F. Determination of benzo[a]pyrene in charcoal grilled meat samples by HPLC with fluorescence detection. Int. J. Food Sci. Nutr. 2005, 56, 581–585. [Google Scholar] [CrossRef]
- Lioy, P.L.; Waldman, J.M.; Greenberg, A.; Harkov, R.; Pietarinen, C. The Total Human Environmental Exposure Study (THEES) to benzo(a)pyrene: Comparison of the inhalation and food pathways. Arch. Environ. Health 1988, 43, 304–312. [Google Scholar] [CrossRef]
- Hattemer-Frey, H.A.; Travis, C.C. Benzo-a-pyrene: Environmental partitioning and human exposure. Toxicol. Ind. Health 1991, 7, 141–157. [Google Scholar] [CrossRef]
- Baan, R.; Grosse, Y.; Straif, K.; Secretan, B.; El Ghissassi, F.; Bouvard, V.; Benbrahim-Tallaa, L.; Guha, N.; Freeman, C.; Galichet, L.; et al. A review of human carcinogens—Part F: Chemical agents and related occupations. Lancet Oncol. 2009, 10, 1143–1144. [Google Scholar] [CrossRef]
- Burchiel, S.W.; Thompson, T.A.; Lauer, F.T.; Oprea, T.I. Activation of dioxin response element (DRE)-associated genes by benzo(a)pyrene 3,6-quinone and benzo(a)pyrene 1,6-quinone in MCF-10A human mammary epithelial cells. Toxicol. Appl. Pharmacol. 2007, 221, 203–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Straif, K.; Baan, R.; Grosse, Y.; Secretan, B.; El Ghissassi, F.; Cogliano, V.; WHO International Agency for Research on Cancer Monograph Working Group. Carcinogenicity of polycyclic aromatic hydrocarbons. Lancet Oncol. 2005, 6, 931–932. [Google Scholar] [CrossRef]
- Huberman, E.; Sachs, L.; Yang, S.K.; Gelboin, V. Identification of mutagenic metabolites of benzo(a)pyrene in mammalian cells. Proc. Natl. Acad. Sci. USA 1976, 73, 607–611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kushman, M.E.; Kabler, S.L.; Fleming, M.H.; Ravoori, S.; Gupta, R.C.; Doehmer, J.; Morrow, C.S.; Townsend, A.J. Expression of human glutathione S-transferase P1 confers resistance to benzo[a]pyrene or benzo[a]pyrene-7,8-dihydrodiol mutagenesis, macromolecular alkylation and formation of stable N2-Gua-BPDE adducts in stably transfected V79MZ cells co-expressing hCYP1A1. Carcinogenesis 2007, 28, 207–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tete, A.; Gallais, I.; Imran, M.; Chevanne, M.; Liamin, M.; Sparfel, L.; Bucher, S.; Burel, A.; Podechard, N.; Appenzeller, B.M.R.; et al. Mechanisms involved in the death of steatotic WIF-B9 hepatocytes co-exposed to benzo[a]pyrene and ethanol: A possible key role for xenobiotic metabolism and nitric oxide. Free Radic. Biol. Med. 2018, 129, 323–337. [Google Scholar] [CrossRef]
- Boei, J.; Vermeulen, S.; Klein, B.; Hiemstra, P.S.; Verhoosel, R.M.; Jennen, D.G.J.; Lahoz, A.; Gmuender, H.; Vrieling, H. Xenobiotic metabolism in differentiated human bronchial epithelial cells. Arch. Toxicol. 2017, 91, 2093–2105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hodges, R.E.; Minich, D.M. Modulation of metabolic detoxification pathways using foods and food-derived components: A scientific review with clinical application. J. Nutr. Metab. 2015, 2015, 760689. [Google Scholar] [CrossRef] [PubMed]
- Uno, S.; Sakai, M.; Fujinari, Y.; Hosono, T.; Seki, T.; Makishima, M. Diallyl trisulfide enhances benzo[a]pyrene-induced CYP1A1 expression and metabolic activation in hepatic HepG2 cells. Anticancer Res. 2019, 39, 2369–2375. [Google Scholar] [CrossRef]
- Hayes, J.D.; Dinkova-Kostova, A.T.; McMahon, M. Cross-Talk between transcription factors AhR and Nrf2: Lessons for cancer chemoprevention from dioxin. Toxicol. Sci. Off. J. Soc. Toxicol. 2009, 111, 199–201. [Google Scholar] [CrossRef]
- Yeager, R.L.; Reisman, S.A.; Aleksunes, L.M.; Klaassen, C.D. Introducing the “TCDD-inducible AhR-Nrf2 gene battery”. Toxicol. Sci. Off. J. Soc. Toxicol. 2009, 111, 238–246. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, S.; Trakooncharoenvit, A.; Nishikawa, M.; Ikushiro, S.; Hara, H. Comprehensive analyses of quercetin conjugates by LC/MS/MS revealed that isorhamnetin-7-O-glucuronide-4’-O-sulfate is a major metabolite in plasma of rats fed with quercetin glucosides. J. Agric. Food Chem. 2019, 67, 4240–4249. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Morand, C.; Demigne, C.; Texier, O.; Regerat, F.; Remesy, C. Bioavailability of rutin and quercetin in rats. FEBS Lett. 1997, 409, 12–16. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Wu, Y.M.; Zhang, P.Y. Protective effects of curcumin and quercetin during benzo(a)pyrene induced lung carcinogenesis in mice. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 1736–1743. [Google Scholar] [PubMed]
- Kozics, K.; Valovicova, Z.; Slamenova, D. Structure of flavonoids influences the degree inhibition of Benzo(a)pyrene-induced DNA damage and micronuclei in HepG2 cells. Neoplasma 2011, 58, 516–524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwarz, D.; Kisselev, P.; Roots, I. CYP1A1 genotype-selective inhibition of benzo[a]pyrene activation by quercetin. Eur. J. Cancer 2005, 41, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Kawai, Y.; Saito, S.; Nishikawa, T.; Ishisaka, A.; Murota, K.; Terao, J. Different profiles of quercetin metabolites in rat plasma: Comparison of two administration methods. Biosci. Biotechnol. Biochem. 2009, 73, 517–523. [Google Scholar] [CrossRef] [Green Version]
- Morand, C.; Crespy, V.; Manach, C.; Besson, C.; Demigne, C.; Remesy, C. Plasma metabolites of quercetin and their antioxidant properties. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1998, 275, R212–R219. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.Z.; Deng, G.; Liang, Q.; Chen, D.F.; Guo, R.; Lai, R.C. Antioxidant activity of quercetin and its glucosides from propolis: A theoretical study. Sci. Rep. 2017, 7, 7543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, L.; Liu, J.; Deng, H.; Gao, C. Benzo[a]pyrene induces autophagic and pyroptotic death simultaneously in HL-7702 human normal liver cells. J. Agric. Food Chem. 2017, 65, 9763–9773. [Google Scholar] [CrossRef] [PubMed]
- Palenik, M.C.; Rodriguez, J.H. Hydrogen-Bonded intermediates and transition states during spontaneous and acid-catalyzed hydrolysis of the carcinogen (+)-anti-BPDE. Phys. Chem. Chem. Phys. 2014, 16, 12684–12687. [Google Scholar] [CrossRef]
- Barnes, J.L.; Zubair, M.; John, K.; Poirier, M.C.; Martin, F.L. Carcinogens and DNA damage. Biochem. Soc. Trans. 2018, 46, 1213–1224. [Google Scholar] [CrossRef] [Green Version]
- Dai, Q.; Ran, C.; Harvey, R.G. Synthesis of adducts of o-quinone metabolites of carcinogenic polycyclic aromatic hydrocarbons with 2’-deoxyribonucleosides. Org. Lett. 2005, 7, 999–1002. [Google Scholar] [CrossRef] [PubMed]
- De Boer, V.C.; Dihal, A.A.; van der Woude, H.; Arts, I.C.; Wolffram, S.; Alink, G.M.; Rietjens, I.M.; Keijer, J.; Hollman, P.C. Tissue distribution of quercetin in rats and pigs. J. Nutr. 2005, 135, 1718–1725. [Google Scholar] [CrossRef] [Green Version]
- Woo, H.D.; Kim, J. Dietary flavonoid intake and risk of stomach and colorectal cancer. World J. Gastroenterol. 2013, 19, 1011–1019. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.M.; Oh, S.J.; Lee, S.Y.; Im, J.H.; Oh, J.M.; Ryu, C.S.; Kwak, H.C.; Lee, J.Y.; Kang, K.W.; Kim, S.K. HepG2 cells as an in vitro model for evaluation of cytochrome P450 induction by xenobiotics. Arch. Pharmacal Res. 2015, 38, 691–704. [Google Scholar] [CrossRef] [PubMed]
- Stavric, B.; Klassen, R. Dietary effects on the uptake of benzo[a]pyrene. Food and chemical toxicology. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 1994, 32, 727–734. [Google Scholar] [CrossRef]
- Petriello, M.C.; Hoffman, J.B.; Morris, A.J.; Hennig, B. Emerging roles of xenobiotic detoxification enzymes in metabolic diseases. Rev. Environ. Health 2017, 32, 105–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kiruthiga, P.V.; Karthikeyan, K.; Archunan, G.; Pandian, S.K.; Devi, K.P. Silymarin prevents benzo(a)pyrene-induced toxicity in Wistar rats by modulating xenobiotic-metabolizing enzymes. Toxicol. Ind. Health 2015, 31, 523–541. [Google Scholar] [CrossRef]
- Smolarek, T.A.; Morgan, S.L.; Moynihan, C.G.; Lee, H.; Harvey, R.G.; Baird, W.M. Metabolism and DNA adduct formation of benzo[a]pyrene and 7,12-dimethylbenz[a]anthracene in fish cell lines in culture. Carcinogenesis 1987, 8, 1501–1509. [Google Scholar] [CrossRef] [PubMed]
- Ashrap, P.; Zheng, G.M.; Wan, Y.; Li, T.; Hu, W.X.; Li, W.J.; Zhang, H.; Zhang, Z.B.; Hu, J.Y. Discovery of a widespread metabolic pathway within and among phenolic xenobiotics. Proc. Natl. Acad. Sci. USA 2017, 114, 6062–6067. [Google Scholar] [CrossRef] [Green Version]
- Sundberg, K.; Dreij, K.; Seidel, A.; Jernstrom, B. Glutathione conjugation and DNA adduct formation of dibenzo[a,l]pyrene and benzo[a]pyrene diol epoxides in V79 cells stably expressing different human glutathione transferases. Chem. Res. Toxicol. 2002, 15, 170–179. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Beyer, A.; Aebersold, R. On the dependency of cellular protein levels on mRNA abundance. Cell 2016, 165, 535–550. [Google Scholar] [CrossRef] [Green Version]
- Bruhn, O.; Lindsay, M.; Wiebel, F.; Kaehler, M.; Nagel, I.; Bohm, R.; Roder, C.; Cascorbi, I. Alternative polyadenylation of ABC transporters of the C-family (ABCC1, ABCC2, ABCC3) and implications on posttranscriptional micro-RNA regulation. Mol. Pharmacol. 2020, 97, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Yim, B.; Kim, J.; Kim, H.; Lee, Y.M. Molecular characterization of ABC transporters in marine ciliate, Euplotes crassus: Identification and response to cadmium and benzo[a]pyrene. Mar. Pollut. Bull. 2017, 124, 725–735. [Google Scholar] [CrossRef]
- Guo, B.Y.; Xu, Z.T.; Yan, X.J.; Buttino, I.; Li, J.J.; Zhou, C.; Qi, P.Z. Novel ABCB1 and ABCC transporters are involved in the detoxification of Benzo(α)pyrene in thick shell mussel, Mytilus coruscus. Front. Mar. Sci. 2020, 7. [Google Scholar] [CrossRef] [Green Version]
- Noda, S.; Harada, N.; Hida, A.; Fujii-Kuriyama, Y.; Motohashi, H.; Yamamoto, M. Gene expression of detoxifying enzymes in AhR and Nrf2 compound null mutant mouse. Biochem. Biophys. Res. Commun. 2003, 303, 105–111. [Google Scholar] [CrossRef]
- Huang, G.; Elferink, C.J. A novel nonconsensus xenobiotic response element capable of mediating aryl hydrocarbon receptor-dependent gene expression. Mol. Pharmacol. 2012, 81, 338–347. [Google Scholar] [CrossRef] [Green Version]
- Wu, K.C.; Cui, J.Y.; Klaassen, C.D. Effect of graded Nrf2 activation on phase-I and -II drug metabolizing enzymes and transporters in mouse liver. PLoS ONE 2012, 7, e39006. [Google Scholar] [CrossRef]
- Pollet, M.; Shaik, S.; Mescher, M.; Frauenstein, K.; Tigges, J.; Braun, S.A.; Sondenheimer, K.; Kaveh, M.; Bruhs, A.; Meller, S.; et al. The AHR represses nucleotide excision repair and apoptosis and contributes to UV-induced skin carcinogenesis. Cell Death Differ. 2018, 25, 1823–1836. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rojo de la Vega, M.; Chapman, E.; Zhang, D.D. NRF2 and the hallmarks of cancer. Cancer Cell 2018, 34, 21–43. [Google Scholar] [CrossRef] [PubMed]
- Sagredo, C.; Ovrebo, S.; Haugen, A.; Fujii-Kuriyama, Y.; Baera, R.; Botnen, I.V.; Mollerup, S. Quantitative analysis of benzo[a]pyrene biotransformation and adduct formation in Ahr knockout mice. Toxicol. Lett. 2006, 167, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Li, D.; He, Z.; Fan, J.; Li, Q.; Liu, X.; Guo, P.; Zhang, H.; Chen, S.; Li, Q.; et al. The effects of Nrf2 knockout on regulation of benzene-induced mouse hematotoxicity. Toxicol. Appl. Pharmacol. 2018, 358, 56–67. [Google Scholar] [CrossRef]
- Jee, S.C.; Kim, M.; Sung, J.S. Modulatory effects of silymarin on benzo[a]pyrene-induced hepatotoxicity. Int. J. Mol. Sci. 2020, 21, 2369. [Google Scholar] [CrossRef] [Green Version]
- Tonelli, C.; Chio, I.I.C.; Tuveson, D.A. Transcriptional Regulation by Nrf2. Antioxid. Redox Signal. 2018, 29, 1727–1745. [Google Scholar] [CrossRef] [Green Version]
- Larigot, L.; Juricek, L.; Dairou, J.; Coumoul, X. AhR signaling pathways and regulatory functions. Biochim. Open 2018, 7, 1–9. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, M.; Jee, S.-C.; Kim, K.-S.; Kim, H.-S.; Yu, K.-N.; Sung, J.-S. Quercetin and Isorhamnetin Attenuate Benzo[a]pyrene-Induced Toxicity by Modulating Detoxification Enzymes through the AhR and NRF2 Signaling Pathways. Antioxidants 2021, 10, 787. https://doi.org/10.3390/antiox10050787
Kim M, Jee S-C, Kim K-S, Kim H-S, Yu K-N, Sung J-S. Quercetin and Isorhamnetin Attenuate Benzo[a]pyrene-Induced Toxicity by Modulating Detoxification Enzymes through the AhR and NRF2 Signaling Pathways. Antioxidants. 2021; 10(5):787. https://doi.org/10.3390/antiox10050787
Chicago/Turabian StyleKim, Min, Seung-Cheol Jee, Kyeong-Seok Kim, Hyung-Sik Kim, Kyoung-Nae Yu, and Jung-Suk Sung. 2021. "Quercetin and Isorhamnetin Attenuate Benzo[a]pyrene-Induced Toxicity by Modulating Detoxification Enzymes through the AhR and NRF2 Signaling Pathways" Antioxidants 10, no. 5: 787. https://doi.org/10.3390/antiox10050787