Role of Oxidative Stress and Nrf2/KEAP1 Signaling in Colorectal Cancer: Mechanisms and Therapeutic Perspectives with Phytochemicals
Abstract
:1. Introduction
2. Oxidative Stress and Lipid Peroxidation in Colon Carcinogenesis
2.1. 4-Hydroxynonenal
2.2. Acrolein
| Lipid Peroxidation Products | Model | Mode of Action | Reference |
|---|---|---|---|
| 4-hydroxynonenal | CaCO-2 | 4-hydroxynonenal ↓ TGF-βI ↓ Apoptosis ↓ | [27] |
| APC+/+ APCMin/+ (colon epithelial cell) | 4-hydroxynonenal metabolism ↑ (APCMin/+) Aldehyde dehydrogenase 1A1, 2, 3A1 ↑ (APCMin/+) Glutathione transferase A4-4 ↑ (APCMin/+) Cystine transporter XCT ↑ (APCMin/+) | [25] | |
| Acrolein | HT29 | Apoptosis ↑ (acrolein 150, 200 μM) DNA adduct ↑ | [36] |
| CCD-841CoN | p-EGFP ↑ (acrolein 5 μM, 8 h) RAS ↑ (acrolein 5 μM, 8 h) p-AKT ↑ (acrolein 5 μM, 8 h) p-ERK ↑ (acrolein 5 μM, 8 h) Cyclin D1 ↑ (acrolein 5 μM, 8 h) | [37] | |
| CRC patients | Acr-dG ↑ (in tumor) Over survival (high Acr-dG > low Acr-dG) | [37] | |
| APCMin/+ | Cocalent adduct with PTEN ↑ pser473Akt ↑ | [38] |
3. Antioxidative Nrf2/KEAP1 Signaling Pathway
3.1. Nrf2
3.2. KEAP1
3.3. Antioxidative Effect by Nrf2/KEAP1 Signal
3.4. Nrf2/KEAP1-Dependent Regulation of Antioxidative Enzymes
3.4.1. Superoxide Dismutase
3.4.2. Catalase
3.4.3. Glutathione Peroxidase
3.4.4. Glutathione
3.4.5. Heme Oxygenase-1
3.4.6. NAD(P)H Quinone Oxidoreductase 1
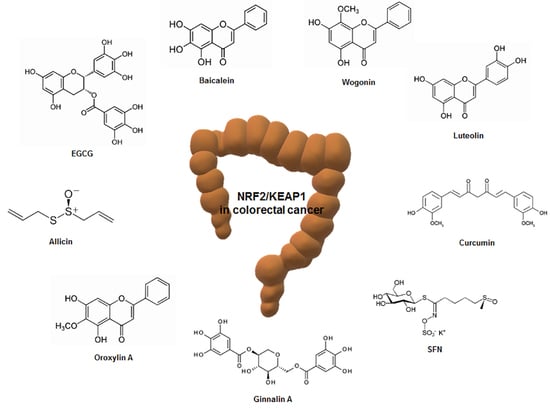
4. Regulation of Nrf2/KEAP1 by Phytochemicals in Colorectal Cancer
4.1. Epigallocatechin-3-Gallate
4.2. Sulforaphane
4.3. Curcumin
4.4. Luteolin
4.5. Allicin
4.6. Resveratrol
4.7. Nobiletin
4.8. Genistein
4.9. Miscellaneous
| Phytochemicals | Effective Dose (Periods) | Experimental Model | Mode of Action | Reference |
|---|---|---|---|---|
| EGCG | 20 mg/kg (6 weeks) | BalB/cA nude mouse | Nrf2 mRNA levels ↑ UGT mRNA levels ↑ Phase II drug metabolizing enzymes ↑ | [128] |
| 5, 10, 20 mg/kg (4 weeks) | BalB/cA nude mouse | Nrf2 protein levels ↑ Nrf2 mRNA levels ↑ UGT1A, UGT1A8 and UGT1A10 mRNA levels ↑ Liver and lung metastasis (20 mg/kg) ↓ | [129] | |
| 12.5 μM | HCT-116 cell | Cell growth ↓ Colony formation ↓ Nrf2 nuclear translocation ↑ LC3B protein levels ↑ Caspase-9 protein levels ↑ | [130] | |
| SFN | 0, 1, 10 μM | HCT-116 | Nrf2 protein levels ↑ HO-1 protein levels ↑ mtDNA/nDNA ratio (p53 KO) ↓ PGC-1α (p53 KO) ↓ | [134] |
| 2.5, 10, 25 mg/kg | p53 wildtype (WT) and p53 knockout (KO) BalB/c nude mouse | Nrf2 protein levels (p53 KO) ↑ Bcl-2 protein levels (p53 KO) ↓ Cytochrome C (p53 KO) ↑ | [134] | |
| 5, 10, 15, 20 μM | HT29 and SW480 cell | Nrf2 protein levels ↑ UGT1A protein levels ↑ Cell proliferation ↓ Cell migration ↓ Colony formation ↓ Apoptosis ↑ | [136] | |
| Curcumin | 1000 mg/kg (single oral dose) | Nrf2 WT and Nrf2 KO C57BL/6J mouse | HO-1 mRNA levels ↑ UGT2B5 mRNA levels ↑ Carbonyl reductase 3 ↑ | [139] |
| Luteolin | 20, 40 μM | HT29 cell | ROS ↑ Cytochrome C protein levels ↑ Bax protein levels ↑ Bcl-2 protein levels ↓ Caspase-3 protein levels ↑ Nrf2 nuclear translocation ↑ Cell proliferation ↓ | [142] |
| 10, 30, 60 μM | HT29 and SNU-407 cell | Methylation of Nrf2 promoter ↓ GCLC protein levels ↑ GSS protein levels ↑ Catalase protein levels ↑ HO-1 protein levels ↑ Bax protein levels ↑ Bcl-2 protein levels ↓ Cleaved caspase-3 protein levels ↑ Cleaved caspase-9 protein levels ↑ Cell viability ↓ | [143] | |
| Allicin | 10 μg/ml | HCT-116 cell | Nrf2 protein levels ↑ Nrf2 promoter activity ↑ Cytochrome C ↑ Bax protein levels ↑ Bcl-2 protein levels ↓ | [146] |
| Resveratrol | 250 ppm | BalB/c mouse (AOM model) | Nrf2 protein levels ↑ HO-1 protein levels ↑ Glutathione reductase mRNA levels ↑ | [151] |
| 20, 50 μM | IPEC-J2 cell (H2O2-induced intestinal barrier injury) | Cell viability ↑ SOD activity ↑ CAT activity ↑ GSH activity ↑ SOD mRNA levels ↑ CAT mRNA levels ↑ HO-1 mRNA levels ↑ Nrf2 mRNA levels ↑ | [152] | |
| Nobiletin | AIN93G diet containing 0.05% nobiletin | CD-1 mouse (AOM/DSS model) | Nrf2 nuclear translocation ↑ Nrf2 protein levels ↑ HO-1 protein levels ↑ NQO-1 protein levels ↑ Cell cycle arrest | [155] |
| Genistein | 2.5 mg/kg body weight (6 weeks) | Wistar rat (dimethylhydrazine-induced colon carcinogenesis) | Nrf2 expression ↑ HO-1 expression ↑ Glutathione levels ↑ NADPH levels ↑ | [156] |
| Baicalein | 40 μM | HCT-116 cell | Nrf2 phosphorylation ↓ | [157] |
| 50, 100, 150, 200 μM | HT29, SW480, HCT-116, and SW620 cell | Cell viability ↓ LC3-II protein levels ↑ (HCT-116) Caspase-3/7 activity ↑ (HCT-116) | [158] | |
| Wogonin | 60 mg/kg (29, 48, 68, 105 days) | C57BL/6 mouse (AOM/DSS model) | Nrf2 nuclear translocation ↑ Nrf2 positive cells ↑ | [160] |
| 25, 50, 100 μM | HCT-116 cell | IL-6, IL-6, IL-1β levels ↓ HO-1 protein levels ↑ NQO1 protein levels ↑ KEAP1 protein levels ↑ Nrf2 promoter activity ↑ | ||
| Oroxylin A | 50, 100, 150 μM | HCT-116 cell | Cell proliferation ↓ Bax protein levels ↑ Bcl-2 protein levels ↓ Nrf2 nuclear translocation ↑ HO-1 protein levels ↑ NQO1 protein levels ↑ | [161] |
| 50, 100, 200 mg/kg | Balb/c mouse | Nrf2 nuclear translocation ↑ | ||
| Ginnalin A | 20, 40, 80 μM | HCT-116, SW480, and SW620 cell | Cell proliferation ↓ S-phase cell cycle arrest Nrf2 mRNA and protein levels ↑ HO-1 mRNA and protein levels ↑ NQO1 mRNA and protein levels ↑ KEAP1 protein levels ↓ | [162] |
5. Clinical Trials of Phytochemicals in Patients with Colorectal Cancer
| Phytochemicals | Dosage (Periods) | Phase | Results | NCT Number | Reference |
|---|---|---|---|---|---|
| EGCG | 900 mg (1 year) | I, II | Chemoprevention | NCT02891538 | - |
| Curcumin | 4 g | I | Chemoprevention | NCT01490996 | - |
| 2, 4 g (30 days) | II | Aberrant crypt foci ↓ | - | [165] | |
| Resveratrol | 20, 80 g | I | Wnt signaling pathway regulation | NCT00256334 | [166] |
| Genistein | 60 mg (2 weeks) | No observation of side effects with/without FOLFOX or FOLFOX-Bevacizumab | NCT01985763 | [167] |
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| Acr-dG | Acrolein-induced DNA damages |
| APC | Adenomatous polyposis coli |
| ARE/EpRE | Antioxidant/electrophile responsive element |
| BTB | Broad complex/Tramtrack/Bric-a-brac |
| bZIP | Basic leucine zipper |
| CAC | Colitis-associated colorectal cancer |
| CBP | CREB-binding protein |
| CD | Crohn’s disease |
| CHD6 | Chromo-ATPase/helicase DNA-binding protein 6 |
| CNC | Cap ’N’ Collar |
| CRC | Colorectal cancer |
| CREB | cAMP response element binding protein |
| Cul3 | Cullin 3 |
| DGR | Double glycine-repeat |
| EGCG | Epigallocatechin-3-Gallate |
| EGFR | Epithelial growth factor receptor |
| ERK | Extracellular-signal-regulated kinase |
| GCLC | Glutamate-cysteine ligase catalytic subunit |
| GCLM | Glutamate-cysteine ligase modifier subunit |
| GPx | Glutathione peroxidase |
| GSH | Glutathione |
| GSK | Glycogen synthase kinase |
| GST | Glutathione-S-transferase |
| HO | Heme oxygenase |
| IBD | Inflammatory bowel disease |
| IVR | Intervening region |
| KEAP1 | Kelch-like-ECH-associated protein 1 |
| NADPH | Nicotinamide adenine dinucleotide phosphate |
| Neh | Nrf2-ECH homology |
| Nrf2 | Nuclear factor erythroid 2-related factor 2 |
| NQO1 | NAD(P)H quinone oxidoreductase 1 |
| PTEN | Phosphatase and tensin homolog |
| PUFA | Polyunsaturated fatty acids |
| ROS | Reactive oxygen species |
| SFN | Sulforaphane |
| sMAF | Small musculoaponeurotic fibrosarcoma protein |
| SOD | Superoxide dismutase |
| TGF-βRI | Transforming growth factor-beta receptor I |
| β-TrCP | β-transducin repeat-containing protein |
| UbcM2 | Ubiquitin-conjugating enzyme |
| UC | Ulcerative colitis |
| UGT | UDP-glucuronosyltransferases |
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef]
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef]
- Bernstein, C.N.; Blanchard, J.F.; Kliewer, E.; Wajda, A. Cancer risk in patients with inflammatory bowel disease: A population-based study. Cancer 2001, 91, 854–862. [Google Scholar] [CrossRef]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive oxygen species in inflammation and tissue injury. Antioxid Redox Signal. 2014, 20, 1126–1167. [Google Scholar] [CrossRef] [Green Version]
- Marnett, L.J. Oxyradicals and DNA damage. Carcinogenesis 2000, 21, 361–370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valko, M.; Rhodes, C.J.; Moncol, J.; Izakovic, M.; Mazur, M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem. Biol. Interact. 2006, 160, 1–40. [Google Scholar] [CrossRef] [PubMed]
- Barrera, G. Oxidative stress and lipid peroxidation products in cancer progression and therapy. ISRN Oncol. 2012, 137289. [Google Scholar] [CrossRef] [Green Version]
- Barrera, G.; Pizzimenti, S.; Dianzani, M.U. Lipid peroxidation: Control of cell proliferation, cell differentiation and cell death. Mol. Asp. Med. 2008, 29, 1–8. [Google Scholar] [CrossRef]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef]
- Kansanen, E.; Jyrkkanen, H.K.; Levonen, A.L. Activation of stress signaling pathways by electrophilic oxidized and nitrated lipids. Free Radic. Biol. Med. 2012, 52, 973–982. [Google Scholar] [CrossRef]
- Cuadrado, A.; Rojo, A.I.; Wells, G.; Hayes, J.D.; Cousin, S.P.; Rumsey, W.L.; Attucks, O.C.; Franklin, S.; Levonen, A.L.; Kensler, T.W.; et al. Therapeutic targeting of the NRF2 and KEAP1 partnership in chronic diseases. Nat. Rev. Drug Discov. 2019, 18, 295–317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arisawa, T.; Tahara, T.; Shibata, T.; Nagasaka, M.; Nakamura, M.; Kamiya, Y.; Fujita, H.; Yoshioka, D.; Okubo, M.; Sakata, M. Nrf2 gene promoter polymorphism is associated with ulcerative colitis in a Japanese population. Hepato Gastroenterol. 2008, 55, 394–397. [Google Scholar]
- Kovac, S.; Angelova, P.R.; Holmstrom, K.M.; Zhang, Y.; Dinkova-Kostova, A.T.; Abramov, A.Y. Nrf2 regulates ROS production by mitochondria and NADPH oxidase. Biochim. Biophys. Acta 2015, 1850, 794–801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, Z.Y.; Shu, L.; Khor, T.O.; Lee, J.H.; Fuentes, F.; Kong, A.N. A perspective on dietary phytochemicals and cancer chemoprevention: Oxidative stress, nrf2, and epigenomics. Top. Curr. Chem. 2013, 329, 133–162. [Google Scholar]
- Lu, J.M.; Lin, P.H.; Yao, Q.; Chen, C. Chemical and molecular mechanisms of antioxidants: Experimental approaches and model systems. J. Cell Mol. Med. 2010, 14, 840–860. [Google Scholar] [CrossRef] [PubMed]
- Xiao, M.; Zhong, H.; Xia, L.; Tao, Y.; Yin, H. Pathophysiology of mitochondrial lipid oxidation: Role of 4-hydroxynonenal (4-HNE) and other bioactive lipids in mitochondria. Free Radic. Biol. Med. 2017, 111, 316–327. [Google Scholar] [CrossRef]
- Ayala, A.; Munoz, M.F.; Arguelles, S. Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid. Med. Cell Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef]
- Dalleau, S.; Baradat, M.; Gueraud, F.; Huc, L. Cell death and diseases related to oxidative stress: 4-hydroxynonenal (HNE) in the balance. Cell Death Differ. 2013, 20, 1615–1630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schaur, R.J. Basic aspects of the biochemical reactivity of 4-hydroxynonenal. Mol. Asp. Med. 2003, 24, 149–159. [Google Scholar] [CrossRef]
- Zhang, H.; Forman, H.J. Signaling pathways involved in phase II gene induction by alpha, beta-unsaturated aldehydes. Toxicol. Ind. Health 2009, 25, 269–278. [Google Scholar] [CrossRef] [Green Version]
- Levy, S.; Jaiswal, A.K.; Forman, H.J. The role of c-Jun phosphorylation in EpRE activation of phase II genes. Free Radic. Biol. Med. 2009, 47, 1172–1179. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.; Vrishni, S.; Singh, B.K.; Rahman, I.; Kakkar, P. Nrf2-ARE stress response mechanism: A control point in oxidative stress-mediated dysfunctions and chronic inflammatory diseases. Free Radic. Res. 2010, 44, 1267–1288. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yang, Y.; Moore, D.R.; Nimmo, S.L.; Lightfoot, S.A.; Huycke, M.M. 4-Hydroxy-2-nonenal mediates genotoxicity and bystander effects caused by Enterococcus faecalis–infected macrophages. Gastroenterology 2012, 142, 543–551.e7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esterbauer, H.; Schaur, R.J.; Zollner, H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic. Biol. Med. 1991, 11, 81–128. [Google Scholar] [CrossRef]
- Baradat, M.; Jouanin, I.; Dalleau, S.; Tache, S.; Gieules, M.; Debrauwer, L.; Canlet, C.; Huc, L.; Dupuy, J.; Pierre, F.H.; et al. 4-Hydroxy-2(E)-nonenal metabolism differs in Apc(+/+) cells and in Apc(Min/+) cells: It may explain colon cancer promotion by heme iron. Chem. Res. Toxicol. 2011, 24, 1984–1993. [Google Scholar] [CrossRef] [PubMed]
- Pierre, F.; Tache, S.; Gueraud, F.; Rerole, A.L.; Jourdan, M.L.; Petit, C. Apc mutation induces resistance of colonic cells to lipoperoxide-triggered apoptosis induced by faecal water from haem-fed rats. Carcinogenesis 2007, 28, 321–327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biasi, F.; Tessitore, L.; Zanetti, D.; Cutrin, J.C.; Zingaro, B.; Chiarpotto, E.; Zarkovic, N.; Serviddio, G.; Poli, G. Associated changes of lipid peroxidation and transforming growth factor beta1 levels in human colon cancer during tumour progression. Gut 2002, 50, 361–367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanner, J. Dietary advanced lipid oxidation endproducts are risk factors to human health. Mol. Nutr. Food Res. 2007, 51, 1094–1101. [Google Scholar] [CrossRef] [PubMed]
- Stevens, J.F.; Maier, C.S. Acrolein: Sources, metabolism, and biomolecular interactions relevant to human health and disease. Mol. Nutr. Food Res. 2008, 52, 7–25. [Google Scholar] [CrossRef] [Green Version]
- Tamura, H.; Kitta, K.; Shibamoto, T. Formation of reactive aldehydes from fatty acids in a iron (2+)/hydrogen peroxide oxidation system. J. Agric. Food Chem. 1991, 39, 439–442. [Google Scholar] [CrossRef]
- Pan, X.; Kaneko, H.; Ushio, H.; Ohshima, T. Oxidation of all-cis-7, 10, 13, 16, 19-docosapentaenoic acid ethyl ester. Hydroperoxide distribution and volatile characterization. Eur. J. Lipid Sci. Technol. 2005, 107, 228–238. [Google Scholar] [CrossRef]
- Tsakadze, N.L.; Srivastava, S.; Awe, S.O.; Adeagbo, A.S.; Bhatnagar, A.; D’Souza, S.E. Acrolein-induced vasomotor responses of rat aorta. Am. J. Physiol. Heart Circ. Physiol. 2003, 285, H727–H734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Myers, C.R.; Myers, J.M.; Kufahl, T.D.; Forbes, R.; Szadkowski, A. The effects of acrolein on the thioredoxin system: Implications for redox-sensitive signaling. Mol. Nutr. Food Res. 2011, 55, 1361–1374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, C.C.; Hsieh, C.W.; Lai, P.H.; Lin, J.B.; Liu, Y.C.; Wung, B.S. Upregulation of endothelial heme oxygenase-1 expression through the activation of the JNK pathway by sublethal concentrations of acrolein. Toxicol. Appl. Pharm. 2006, 214, 244–252. [Google Scholar] [CrossRef]
- Tirumalai, R.; Rajesh Kumar, T.; Mai, K.H.; Biswal, S. Acrolein causes transcriptional induction of phase II genes by activation of Nrf2 in human lung type II epithelial (A549) cells. Toxicol. Lett. 2002, 132, 27–36. [Google Scholar] [CrossRef]
- Pan, J.; Keffer, J.; Emami, A.; Ma, X.; Lan, R.; Goldman, R.; Chung, F.L. Acrolein-derived DNA adduct formation in human colon cancer cells: Its role in apoptosis induction by docosahexaenoic acid. Chem. Res. Toxicol. 2009, 22, 798–806. [Google Scholar] [CrossRef] [Green Version]
- Tsai, H.-C.; Tsou, H.-H.; Lin, C.-C.; Chen, S.-C.; Cheng, H.-W.; Liu, T.-Y.; Chen, W.-S.; Jiang, J.-K.; Yang, S.-H.; Chang, S.-C. Acrolein Contributes to Human Colorectal Tumorigenesis Through Activation of RAS/MAPK Pathway. Res. Sq. 2021. [Google Scholar] [CrossRef]
- Al-Salihi, M.; Reichert, E.; Fitzpatrick, F.A. Influence of myeloperoxidase on colon tumor occurrence in inflamed versus non-inflamed colons of Apc(Min/+) mice. Redox Biol. 2015, 6, 218–225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Itoh, K.; Igarashi, K.; Hayashi, N.; Nishizawa, M.; Yamamoto, M. Cloning and characterization of a novel erythroid cell-derived CNC family transcription factor heterodimerizing with the small Maf family proteins. Mol. Cell Biol. 1995, 15, 4184–4193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moi, P.; Chan, K.; Asunis, I.; Cao, A.; Kan, Y.W. Isolation of NF-E2-related factor 2 (Nrf2), a NF-E2-like basic leucine zipper transcriptional activator that binds to the tandem NF-E2/AP1 repeat of the beta-globin locus control region. Proc. Natl. Acad. Sci. USA 1994, 91, 9926–9930. [Google Scholar] [CrossRef] [Green Version]
- Zhang, D.D. Mechanistic studies of the Nrf2-Keap1 signaling pathway. Drug Metab. Rev. 2006, 38, 769–789. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.D.; Lo, S.-C.; Cross, J.V.; Templeton, D.J.; Hannink, M. Keap1 is a redox-regulated substrate adaptor protein for a Cul3-dependent ubiquitin ligase complex. Mol. Cell. Biol. 2004, 24, 10941–10953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McMahon, M.; Thomas, N.; Itoh, K.; Yamamoto, M.; Hayes, J.D. Dimerization of substrate adaptors can facilitate cullin-mediated ubiquitylation of proteins by a “tethering” mechanism: A two-site interaction model for the Nrf2-Keap1 complex. J. Biol. Chem. 2006, 281, 24756–24768. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, A.; Kang, M.-I.; Okawa, H.; Ohtsuji, M.; Zenke, Y.; Chiba, T.; Igarashi, K.; Yamamoto, M. Oxidative stress sensor Keap1 functions as an adaptor for Cul3-based E3 ligase to regulate proteasomal degradation of Nrf2. Mol. Cell. Biol. 2004, 24, 7130–7139. [Google Scholar] [CrossRef] [Green Version]
- Itoh, K.; Wakabayashi, N.; Katoh, Y.; Ishii, T.; Igarashi, K.; Engel, J.D.; Yamamoto, M. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev. 1999, 13, 76–86. [Google Scholar] [CrossRef] [Green Version]
- Katoh, Y.; Itoh, K.; Yoshida, E.; Miyagishi, M.; Fukamizu, A.; Yamamoto, M. Two domains of Nrf2 cooperatively bind CBP, a CREB binding protein, and synergistically activate transcription. Genes Cells 2001, 6, 857–868. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liu, K.; Geng, M.; Gao, P.; Wu, X.; Hai, Y.; Li, Y.; Li, Y.; Luo, L.; Hayes, J.D. RXRα inhibits the NRF2-ARE signaling pathway through a direct interaction with the Neh7 domain of NRF2. Cancer Res. 2013, 73, 3097–3108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chowdhry, S.; Zhang, Y.; McMahon, M.; Sutherland, C.; Cuadrado, A.; Hayes, J.D. Nrf2 is controlled by two distinct β-TrCP recognition motifs in its Neh6 domain, one of which can be modulated by GSK-3 activity. Oncogene 2013, 32, 3765–3781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steel, R.; Cowan, J.; Payerne, E.; O’Connell, M.A.; Searcey, M. Anti-inflammatory Effect of a Cell-Penetrating Peptide Targeting the Nrf2/Keap1 Interaction. ACS Med. Chem. Lett. 2012, 3, 407–410. [Google Scholar] [CrossRef]
- Nioi, P.; Nguyen, T.; Sherratt, P.J.; Pickett, C.B. The carboxy-terminal Neh3 domain of Nrf2 is required for transcriptional activation. Mol. Cell Biol. 2005, 25, 10895–10906. [Google Scholar] [CrossRef] [Green Version]
- Motohashi, H.; Yamamoto, M. Nrf2-Keap1 defines a physiologically important stress response mechanism. Trends Mol. Med. 2004, 10, 549–557. [Google Scholar] [CrossRef] [PubMed]
- Itoh, K.; Chiba, T.; Takahashi, S.; Ishii, T.; Igarashi, K.; Katoh, Y.; Oyake, T.; Hayashi, N.; Satoh, K.; Hatayama, I. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem. Biophys. Res. Commun. 1997, 236, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Jaramillo, M.C.; Zhang, D.D. The emerging role of the Nrf2-Keap1 signaling pathway in cancer. Genes Dev. 2013, 27, 2179–2191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, K.; Han, X.D.; Kan, Y.W. An important function of Nrf2 in combating oxidative stress: Detoxification of acetaminophen. Proc. Natl. Acad. Sci. USA 2001, 98, 4611–4616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Talalay, P.; Dinkova-Kostova, A.T.; Holtzclaw, W.D. Importance of phase 2 gene regulation in protection against electrophile and reactive oxygen toxicity and carcinogenesis. Adv. Enzym. Regul. 2003, 43, 121–134. [Google Scholar] [CrossRef]
- Lau, A.; Villeneuve, N.F.; Sun, Z.; Wong, P.K.; Zhang, D.D. Dual roles of Nrf2 in cancer. Pharm. Res. 2008, 58, 262–270. [Google Scholar] [CrossRef]
- Ooi, B.K.; Chan, K.G.; Goh, B.H.; Yap, W.H. The Role of Natural Products in Targeting Cardiovascular Diseases via Nrf2 Pathway: Novel Molecular Mechanisms and Therapeutic Approaches. Front. Pharm. 2018, 9, 1308. [Google Scholar] [CrossRef] [Green Version]
- Zipper, L.M.; Mulcahy, R.T. The Keap1 BTB/POZ dimerization function is required to sequester Nrf2 in cytoplasm. J. Biol. Chem. 2002, 277, 36544–36552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, D.D.; Hannink, M. Distinct cysteine residues in Keap1 are required for Keap1-dependent ubiquitination of Nrf2 and for stabilization of Nrf2 by chemopreventive agents and oxidative stress. Mol. Cell Biol. 2003, 23, 8137–8151. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Zhang, D.; Hannink, M.; Beamer, L.J. Crystal structure of the Kelch domain of human Keap1. J. Biol. Chem. 2004, 279, 54750–54758. [Google Scholar] [CrossRef] [Green Version]
- Taguchi, K.; Motohashi, H.; Yamamoto, M. Molecular mechanisms of the Keap1-Nrf2 pathway in stress response and cancer evolution. Genes Cells 2011, 16, 123–140. [Google Scholar] [CrossRef]
- Sihvola, V.; Levonen, A.-L. Keap1 as the redox sensor of the antioxidant response. Arch. Biochem. Biophys. 2017, 617, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Dinkova-Kostova, A.T.; Holtzclaw, W.D.; Cole, R.N.; Itoh, K.; Wakabayashi, N.; Katoh, Y.; Yamamoto, M.; Talalay, P. Direct evidence that sulfhydryl groups of Keap1 are the sensors regulating induction of phase 2 enzymes that protect against carcinogens and oxidants. Proc. Natl. Acad. Sci. USA 2002, 99, 11908–11913. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, M.; Kensler, T.W.; Motohashi, H. The KEAP1-NRF2 system: A thiol-based sensor-effector apparatus for maintaining redox homeostasis. Physiol. Rev. 2018, 98, 1169–1203. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, T.; Muramatsu, A.; Saito, R.; Iso, T.; Shibata, T.; Kuwata, K.; Kawaguchi, S.I.; Iwawaki, T.; Adachi, S.; Suda, H.; et al. Molecular Mechanism of Cellular Oxidative Stress Sensing by Keap1. Cell Rep. 2019, 28, 746–758.e4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dhakshinamoorthy, S.; Long II, D.J.; Jaiswal, A.K. Antioxidant regulation of genes encoding enzymes that detoxify xenobiotics and carcinogens. Curr. Top. Cell. Regul. 2001, 36, 201–216. [Google Scholar]
- Lee, J.M.; Moehlenkamp, J.D.; Hanson, J.M.; Johnson, J.A. Nrf2-dependent activation of the antioxidant responsive element by tert-butylhydroquinone is independent of oxidative stress in IMR-32 human neuroblastoma cells. Biochem. Biophys. Res. Commun. 2001, 280, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Alam, J.; Wicks, C.; Stewart, D.; Gong, P.; Touchard, C.; Otterbein, S.; Choi, A.M.; Burow, M.E.; Tou, J. Mechanism of heme oxygenase-1 gene activation by cadmium in MCF-7 mammary epithelial cells. Role of p38 kinase and Nrf2 transcription factor. J. Biol. Chem. 2000, 275, 27694–27702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raghunath, A.; Sundarraj, K.; Arfuso, F.; Sethi, G.; Perumal, E. Dysregulation of Nrf2 in Hepatocellular Carcinoma: Role in Cancer Progression and Chemoresistance. Cancers 2018, 10, 481. [Google Scholar] [CrossRef] [Green Version]
- Ighodaro, O.; Akinloye, O. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alex. J. Med. 2018, 54, 287–293. [Google Scholar] [CrossRef] [Green Version]
- Waris, G.; Ahsan, H. Reactive oxygen species: Role in the development of cancer and various chronic conditions. J. Carcinog 2006, 5, 14. [Google Scholar] [CrossRef]
- Fridovich, I. Superoxide radical and superoxide dismutases. Annu. Rev. Biochem. 1995, 64, 97–112. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant. Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Karuppanapandian, T.; Moon, J.-C.; Kim, C.; Manoharan, K.; Kim, W. Reactive oxygen species in plants: Their generation, signal transduction, and scavenging mechanisms. Aust. J. Crop. Sci. 2011, 5, 709–725. [Google Scholar]
- Bafana, A.; Dutt, S.; Kumar, A.; Kumar, S.; Ahuja, P.S. The basic and applied aspects of superoxide dismutase. J. Mol. Catal. B Enzym. 2011, 68, 129–138. [Google Scholar] [CrossRef]
- Oberley, T.D. Mitochondria, manganese superoxide dismutase, and cancer. Antioxid Redox Signal. 2004, 6, 483–487. [Google Scholar] [CrossRef]
- Papa, L.; Manfredi, G.; Germain, D. SOD1, an unexpected novel target for cancer therapy. Genes Cancer 2014, 5, 15–21. [Google Scholar] [CrossRef] [Green Version]
- Krishnamurthy, P.; Wadhwani, A. Antioxidant enzymes and human health. Antioxid. Enzym. 2012, 1, 3–18. [Google Scholar]
- Robbins, D.; Zhao, Y. Manganese superoxide dismutase in cancer prevention. Antioxid Redox Signal. 2014, 20, 1628–1645. [Google Scholar] [CrossRef] [Green Version]
- Radi, R.; Turrens, J.F.; Chang, L.Y.; Bush, K.M.; Crapo, J.D.; Freeman, B.A. Detection of catalase in rat heart mitochondria. J. Biol. Chem. 1991, 266, 22028–22034. [Google Scholar] [CrossRef]
- Kurutas, E.B. The importance of antioxidants which play the role in cellular response against oxidative/nitrosative stress: Current state. Nutr. J. 2015, 15, 1–22. [Google Scholar] [CrossRef] [Green Version]
- Dröge, W. Free radicals in the physiological control of cell function. Physiol. Rev. 2002, 82, 47–95. [Google Scholar] [CrossRef] [PubMed]
- Ercal, N.; Gurer-Orhan, H.; Aykin-Burns, N. Toxic metals and oxidative stress part I: Mechanisms involved in metal-induced oxidative damage. Curr. Top. Med. Chem. 2001, 1, 529–539. [Google Scholar] [CrossRef]
- Glorieux, C.; Calderon, P.B. Catalase, a remarkable enzyme: Targeting the oldest antioxidant enzyme to find a new cancer treatment approach. Biol. Chem. 2017, 398, 1095–1108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Góth, L.; Rass, P.; Páy, A. Catalase enzyme mutations and their association with diseases. Mol. Diagn. 2004, 8, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Morón, Ú.M.; Castilla-Cortázar, I. Protection against oxidative stress and “IGF-I deficiency conditions”. Antioxid. Enzym. 2012, 89. [Google Scholar] [CrossRef] [Green Version]
- Drevet, J. Glutathione peroxidases expression in the mammalian epididymis and vas deferens. Int. J. Androl. Suppl. 2000, 23, 427–461. [Google Scholar]
- Drevet, J.R. The antioxidant glutathione peroxidase family and spermatozoa: A complex story. Mol. Cell. Endocrinol. 2006, 250, 70–79. [Google Scholar] [CrossRef]
- Burk, R.F.; Olson, G.E.; Winfrey, V.P.; Hill, K.E.; Yin, D. Glutathione peroxidase-3 produced by the kidney binds to a population of basement membranes in the gastrointestinal tract and in other tissues. Am. J. Physiol. Gastrointest. Liver Physiol. 2011, 301, G32–G38. [Google Scholar] [CrossRef] [Green Version]
- Chabory, E.; Damon, C.; Lenoir, A.; Kauselmann, G.; Kern, H.; Zevnik, B.; Garrel, C.; Saez, F.; Cadet, R.; Henry-Berger, J. Epididymis seleno-independent glutathione peroxidase 5 maintains sperm DNA integrity in mice. J. Clin. Investig. 2009, 119, 2074–2085. [Google Scholar] [CrossRef]
- Forgione, M.A.; Weiss, N.; Heydrick, S.; Cap, A.; Klings, E.S.; Bierl, C.; Eberhardt, R.T.; Farber, H.W.; Loscalzo, J. Cellular glutathione peroxidase deficiency and endothelial dysfunction. Am. J. Physiol. Heart Circ. Physiol. 2002, 282, H1255–H1261. [Google Scholar] [CrossRef]
- Paulsen, C.E.; Carroll, K.S. Orchestrating redox signaling networks through regulatory cysteine switches. ACS Chem. Biol. 2010, 5, 47–62. [Google Scholar] [CrossRef] [Green Version]
- Hansen, R.D.; Krath, B.N.; Frederiksen, K.; Tjønneland, A.; Overvad, K.; Roswall, N.; Loft, S.; Dragsted, L.O.; Vogel, U.; Raaschou-Nielsen, O. GPX1 Pro198Leu polymorphism, erythrocyte GPX activity, interaction with alcohol consumption and smoking, and risk of colorectal cancer. Mutat. Res. Fundam. Mol. Mech. Mutagen. 2009, 664, 13–19. [Google Scholar] [CrossRef]
- Forman, H.J.; Zhang, H.; Rinna, A. Glutathione: Overview of its protective roles, measurement, and biosynthesis. Mol. Asp. Med. 2009, 30, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Kerksick, C.; Willoughby, D. The antioxidant role of glutathione and N-acetyl-cysteine supplements and exercise-induced oxidative stress. J. Int. Soc. Sports Nutr. 2005, 2, 38–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aquilano, K.; Baldelli, S.; Ciriolo, M.R. Glutathione: New roles in redox signaling for an old antioxidant. Front. Pharm. 2014, 5, 196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lubos, E.; Loscalzo, J.; Handy, D.E. Glutathione peroxidase-1 in health and disease: From molecular mechanisms to therapeutic opportunities. Antioxid. Redox Signal. 2011, 15, 1957–1997. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, S.C. Glutathione synthesis. Biochim. Biophys. Acta 2013, 1830, 3143–3153. [Google Scholar] [CrossRef] [Green Version]
- Lu, S.C. Regulation of glutathione synthesis. Mol. Asp. Med. 2009, 30, 42–59. [Google Scholar] [CrossRef] [Green Version]
- Pinkus, R.; Weiner, L.M.; Daniel, V. Role of oxidants and antioxidants in the induction of AP-1, NF-κB, and glutathione S-transferase gene expression. J. Biol. Chem. 1996, 271, 13422–13429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Townsend, D.M.; Tew, K.D.; Tapiero, H. The importance of glutathione in human disease. Biomed. Pharm. 2003, 57, 145–155. [Google Scholar] [CrossRef]
- Dickinson, D.A.; Iles, K.E.; Zhang, H.; Blank, V.; Forman, H.J. Curcumin alters EpRE and AP-1 binding complexes and elevates glutamate-cysteine ligase gene expression. FASEB J. 2003, 17, 1–26. [Google Scholar] [CrossRef]
- Brooks, J.D.; Paton, V.G.; Vidanes, G. Potent induction of phase 2 enzymes in human prostate cells by sulforaphane. Cancer Epidemiol. Prev. Biomark. 2001, 10, 949–954. [Google Scholar]
- Gozzelino, R.; Jeney, V.; Soares, M.P. Mechanisms of cell protection by heme oxygenase-1. Annu. Rev. Pharm. Toxicol. 2010, 50, 323–354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tafani, M.; Sansone, L.; Limana, F.; Arcangeli, T.; De Santis, E.; Polese, M.; Fini, M.; Russo, M.A. The Interplay of Reactive Oxygen Species, Hypoxia, Inflammation, and Sirtuins in Cancer Initiation and Progression. Oxid. Med. Cell Longev. 2016, 2016, 3907147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grochot-Przeczek, A.; Dulak, J.; Jozkowicz, A. Haem oxygenase-1: Non-canonical roles in physiology and pathology. Clin. Sci. 2012, 122, 93–103. [Google Scholar] [CrossRef] [Green Version]
- Converso, D.P.; Taillé, C.; Carreras, M.C.; Jaitovich, A.; Poderoso, J.J.; Boczkowski, J.; Converso, D.P.; Taillé, C.; Carreras, M.C.; Jaitovich, A. HO-1 is located in liver mitochondria and modulates mitochondrial heme content and metabolism. FASEB J. 2006, 20, 1236–1238. [Google Scholar] [CrossRef] [Green Version]
- Lin, Q.; Weis, S.; Yang, G.; Weng, Y.-H.; Helston, R.; Rish, K.; Smith, A.; Bordner, J.; Polte, T.; Gaunitz, F. Heme oxygenase-1 protein localizes to the nucleus and activates transcription factors important in oxidative stress. J. Biol. Chem. 2007, 282, 20621–20633. [Google Scholar] [CrossRef] [Green Version]
- Dennery, P.A. Signaling function of heme oxygenase proteins. Antioxid. Redox Signal. 2014, 20, 1743–1753. [Google Scholar] [CrossRef] [Green Version]
- Alam, J.; Cook, J.L. How many transcription factors does it take to turn on the heme oxygenase-1 gene? Am. J. Respir. Cell Mol. Biol. 2007, 36, 166–174. [Google Scholar] [CrossRef]
- Araujo, J.A.; Zhang, M.; Yin, F. Heme oxygenase-1, oxidation, inflammation, and atherosclerosis. Front. Pharm. 2012, 3, 119. [Google Scholar] [CrossRef] [Green Version]
- Kim, E.H.; Kim, S.J.; Na, H.K.; Han, W.; Kim, N.J.; Suh, Y.G.; Surh, Y.J. 15-Deoxy-Delta(12,14)-prostaglandin J2 Upregulates VEGF Expression via NRF2 and Heme Oxygenase-1 in Human Breast Cancer Cells. Cells 2021, 10, 526. [Google Scholar] [CrossRef]
- Nitti, M.; Piras, S.; Marinari, U.M.; Moretta, L.; Pronzato, M.A.; Furfaro, A.L. HO-1 Induction in Cancer Progression: A Matter of Cell Adaptation. Antioxidants 2017, 6, 29. [Google Scholar] [CrossRef] [PubMed]
- Was, H.; Dulak, J.; Jozkowicz, A. Heme oxygenase-1 in tumor biology and therapy. Curr. Drug Targets 2010, 11, 1551–1570. [Google Scholar] [CrossRef] [PubMed]
- Terai, K.; Dong, G.-Z.; Oh, E.-T.; Park, M.-T.; Gu, Y.; Song, C.W.; Park, H.J. Cisplatin enhances the anticancer effect of β-lapachone by upregulating NQO1. Anti-Cancer Drugs 2009, 20, 901–909. [Google Scholar] [CrossRef]
- Ross, D.; Siegel, D. Functions of NQO1 in Cellular Protection and CoQ10 Metabolism and its Potential Role as a Redox Sensitive Molecular Switch. Front. Physiol 2017, 8, 595. [Google Scholar] [CrossRef] [PubMed]
- Jo, H.S.; Kim, D.S.; Ahn, E.H.; Kim, D.W.; Shin, M.J.; Cho, S.B.; Park, J.H.; Lee, C.H.; Yeo, E.J.; Choi, Y.J.; et al. Protective effects of Tat-NQO1 against oxidative stress-induced HT-22 cell damage, and ischemic injury in animals. BMB Rep. 2016, 49, 617–622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakamura, Y.; Ohigashi, H.; Masuda, S.; Murakami, A.; Morimitsu, Y.; Kawamoto, Y.; Osawa, T.; Imagawa, M.; Uchida, K. Redox regulation of glutathione S-transferase induction by benzyl isothiocyanate: Correlation of enzyme induction with the formation of reactive oxygen intermediates. Cancer Res. 2000, 60, 219–225. [Google Scholar]
- Oh, E.T.; Park, H.J. Implications of NQO1 in cancer therapy. Bmb Rep. 2015, 48, 609–617. [Google Scholar] [CrossRef] [Green Version]
- Krajka-Kuźniak, V.; Paluszczak, J.; Baer-Dubowska, W. The Nrf2-ARE signaling pathway: An update on its regulation and possible role in cancer prevention and treatment. Pharmacol. Rep. 2017, 69, 393–402. [Google Scholar] [CrossRef]
- Bauer, A.K.; Faiola, B.; Abernethy, D.J.; Marchan, R.; Pluta, L.J.; Wong, V.A.; Roberts, K.; Jaiswal, A.K.; Gonzalez, F.J.; Butterworth, B.E. Genetic susceptibility to benzene-induced toxicity: Role of NADPH: Quinone oxidoreductase-1. Cancer Res. 2003, 63, 929–935. [Google Scholar]
- Van De Wier, B.; Koek, G.H.; Bast, A.; Haenen, G.R. The potential of flavonoids in the treatment of non-alcoholic fatty liver disease. Crit. Rev. Food Sci. Nutr. 2017, 57, 834–855. [Google Scholar] [CrossRef] [PubMed]
- Kwak, M.-K.; Kensler, T.W. Targeting NRF2 signaling for cancer chemoprevention. Toxicol. Appl. Pharmacol. 2010, 244, 66–76. [Google Scholar] [CrossRef] [Green Version]
- Bhattacharjee, S.; Dashwood, R.H. Epigenetic Regulation of NRF2/KEAP1 by Phytochemicals. Antioxidants 2020, 9, 865. [Google Scholar] [CrossRef] [PubMed]
- Lambert, J.D.; Yang, C.S. Mechanisms of cancer prevention by tea constituents. J. Nutr. 2003, 133, 3262S–3267S. [Google Scholar] [CrossRef]
- Park, O.J.; Surh, Y.-J. Chemopreventive potential of epigallocatechin gallate and genistein: Evidence from epidemiological and laboratory studies. Toxicol. Lett. 2004, 150, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Wubetu, G.Y.; Shimada, M.; Morine, Y.; Ikemoto, T.; Ishikawa, D.; Iwahashi, S.; Yamada, S.; Saito, Y.; Arakawa, Y.; Imura, S. Epigallocatechin gallate hinders human hepatoma and colon cancer sphere formation. J. Gastroenterol. Hepatol. 2016, 31, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.-H.; Li, Y.-Q.; Yang, X.-Y. Protective effects of epigallocatechin gallate on colon preneoplastic lesions induced by 2-amino-3-methylimidazole(4,5-f)quinoloine in mice. Mol. Med. 2008, 14, 590–598. [Google Scholar] [CrossRef]
- Yuan, J.-H.; Li, Y.-Q.; Yang, X.-Y. Inhibition of epigallocatechin gallate on orthotopic colon cancer by upregulating the Nrf2-UGT1A signal pathway in nude mice. Pharmacology 2007, 80, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Enkhbat, T.; Nishi, M.; Yoshikawa, K.; Jun, H.; Tokunaga, T.; Takasu, C.; Kashihara, H.; Ishikawa, D.; Tominaga, M.; Shimada, M. Epigallocatechin-3-gallate Enhances Radiation Sensitivity in Colorectal Cancer Cells Through Nrf2 Activation and Autophagy. Anticancer Res. 2018, 38, 6247–6252. [Google Scholar] [CrossRef]
- Yin, T.-F.; Wang, M.; Qing, Y.; Lin, Y.-M.; Wu, D. Research progress on chemopreventive effects of phytochemicals on colorectal cancer and their mechanisms. World J. Gastroenterol. 2016, 22, 7058. [Google Scholar] [CrossRef]
- Vuong, L.D.; Nguyen, Q.N.; Truong, V.-L. Anti-inflammatory and anti-oxidant effects of combination between sulforaphane and acetaminophen in LPS-stimulated RAW 264.7 macrophage cells. Immunopharmacol. Immunotoxicol. 2019, 41, 413–419. [Google Scholar] [CrossRef]
- Thimmulappa, R.K.; Mai, K.H.; Srisuma, S.; Kensler, T.W.; Yamamoto, M.; Biswal, S. Identification of Nrf2-regulated genes induced by the chemopreventive agent sulforaphane by oligonucleotide microarray. Cancer Res. 2002, 62, 5196–5203. [Google Scholar]
- Gwon, Y.; Oh, J.; Kim, J.-S. Sulforaphane induces colorectal cancer cell proliferation through Nrf2 activation in a p53-dependent manner. Appl. Biol. Chem. 2020, 63, 1–11. [Google Scholar] [CrossRef]
- Faraonio, R.; Vergara, P.; Di Marzo, D.; Pierantoni, M.G.; Napolitano, M.; Russo, T.; Cimino, F. p53 suppresses the Nrf2-dependent transcription of antioxidant response genes. J. Biol. Chem. 2006, 281, 39776–39784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hao, Q.; Wang, M.; Sun, N.X.; Zhu, C.; Lin, Y.M.; Li, C.; Liu, F.; Zhu, W.W. Sulforaphane suppresses carcinogenesis of colorectal cancer through the ERK/Nrf2-UDP glucuronosyltransferase 1A metabolic axis activation. Oncol. Rep. 2020, 43, 1067–1080. [Google Scholar] [CrossRef]
- Lin, S.-S.; Lai, K.-C.; Hsu, S.-C.; Yang, J.-S.; Kuo, C.-L.; Lin, J.-P.; Ma, Y.-S.; Wu, C.-C.; Chung, J.-G. Curcumin inhibits the migration and invasion of human A549 lung cancer cells through the inhibition of matrix metalloproteinase-2 and-9 and Vascular Endothelial Growth Factor (VEGF). Cancer Lett. 2009, 285, 127–133. [Google Scholar] [CrossRef]
- Sharma, R.; Gescher, A.; Steward, W. Curcumin: The story so far. Eur. J. Cancer 2005, 41, 1955–1968. [Google Scholar] [CrossRef] [PubMed]
- Shen, G.; Xu, C.; Hu, R.; Jain, M.R.; Gopalkrishnan, A.; Nair, S.; Huang, M.T.; Chan, J.Y.; Kong, A.N. Modulation of nuclear factor E2-related factor 2-mediated gene expression in mice liver and small intestine by cancer chemopreventive agent curcumin. Mol. Cancer 2006, 5, 39–51. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.; Shi, R.; Wang, X.; Shen, H.-M. Luteolin, a flavonoid with potential for cancer prevention and therapy. Curr. Cancer Drug Targets 2008, 8, 634–646. [Google Scholar] [CrossRef] [PubMed]
- López-Lázaro, M. Distribution and biological activities of the flavonoid luteolin. Mini Rev. Med. Chem. 2009, 9, 31–59. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Liu, B.F.; Xie, F.J.; Yang, W.L.; Cao, N. Luteolin induces mitochondrial apoptosis in HT29 cells by inhibiting the Nrf2/ARE signaling pathway. Exp. Med. 2020, 19, 2179–2187. [Google Scholar] [CrossRef] [Green Version]
- Kang, K.A.; Piao, M.J.; Hyun, Y.J.; Zhen, A.X.; Cho, S.J.; Ahn, M.J.; Yi, J.M.; Hyun, J.W. Luteolin promotes apoptotic cell death via upregulation of Nrf2 expression by DNA demethylase and the interaction of Nrf2 with p53 in human colon cancer cells. Exp. Mol. Med. 2019, 51, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Zhu, B.; Zhao, L.; Liu, Y.; Zhao, F.; Feng, J.; Jin, Y.; Sun, J.; Geng, R.; Wei, Y. Allicin inhibits proliferation and invasion in vitro and in vivo via SHP-1-mediated STAT3 signaling in cholangiocarcinoma. Cell. Physiol. Biochem. 2018, 47, 641–653. [Google Scholar] [CrossRef] [PubMed]
- Mikaili, P.; Maadirad, S.; Moloudizargari, M.; Aghajanshakeri, S.; Sarahroodi, S. Therapeutic uses and pharmacological properties of garlic, shallot, and their biologically active compounds. Iran. J. Basic Med. Sci. 2013, 16, 1031. [Google Scholar] [PubMed]
- Bat-Chen, W.; Golan, T.; Peri, I.; Ludmer, Z.; Schwartz, B. Allicin purified from fresh garlic cloves induces apoptosis in colon cancer cells via Nrf2. Nutr. Cancer 2010, 62, 947–957. [Google Scholar] [CrossRef]
- Kim, S.; Lee, H.G.; Park, S.A.; Kundu, J.K.; Keum, Y.S.; Cha, Y.N.; Na, H.K.; Surh, Y.J. Keap1 cysteine 288 as a potential target for diallyl trisulfide-induced Nrf2 activation. PLoS ONE 2014, 9, e85984. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, D.Y.; Yun, S.M.; Song, M.Y.; Jung, K.; Kim, E.H. Cyanidin Chloride Induces Apoptosis by Inhibiting NF-kappaB Signaling through Activation of Nrf2 in Colorectal Cancer Cells. Antioxidants 2020, 9, 285. [Google Scholar] [CrossRef] [Green Version]
- Berman, A.Y.; Motechin, R.A.; Wiesenfeld, M.Y.; Holz, M.K. The therapeutic potential of resveratrol: A review of clinical trials. NPJ Precis Oncol. 2017, 1, 35–43. [Google Scholar] [CrossRef] [Green Version]
- Shankar, S.; Singh, G.; Srivastava, R.K. Chemoprevention by resveratrol: Molecular mechanisms and therapeutic potential. Front. Biosci. 2007, 12, 4839–4854. [Google Scholar] [CrossRef] [Green Version]
- Chiou, Y.-S.; Tsai, M.-L.; Nagabhushanam, K.; Wang, Y.-J.; Wu, C.-H.; Ho, C.-T.; Pan, M.-H. Pterostilbene is more potent than resveratrol in preventing azoxymethane (AOM)-induced colon tumorigenesis via activation of the NF-E2-related factor 2 (Nrf2)-mediated antioxidant signaling pathway. J. Agric. Food Chem. 2011, 59, 2725–2733. [Google Scholar] [CrossRef]
- Zhuang, Y.; Wu, H.; Wang, X.; He, J.; He, S.; Yin, Y. Resveratrol Attenuates Oxidative Stress-Induced Intestinal Barrier Injury through PI3K/Akt-Mediated Nrf2 Signaling Pathway. Oxid. Med. Cell Longev. 2019, 2019, 7591840. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Pan, M.-H.; Lo, C.-Y.; Tan, D.; Wang, Y.; Shahidi, F.; Ho, C.-T. Chemistry and health effects of polymethoxyflavones and hydroxylated polymethoxyflavones. J. Funct. Foods 2009, 1, 2–12. [Google Scholar] [CrossRef]
- Ashrafizadeh, M.; Zarrabi, A.; Saberifar, S.; Hashemi, F.; Hushmandi, K.; Hashemi, F.; Moghadam, E.R.; Mohammadinejad, R.; Najafi, M.; Garg, M. Nobiletin in cancer therapy: How this plant derived-natural compound targets various oncogene and onco-suppressor pathways. Biomedicines 2020, 8, 110. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Song, M.; Gao, Z.; Sun, Y.; Wang, M.; Li, F.; Zheng, J.; Xiao, H. Nobiletin and its colonic metabolites suppress colitis-associated colon carcinogenesis by down-regulating iNOS, inducing antioxidative enzymes and arresting cell cycle progression. J. Nutr. Biochem. 2017, 42, 17–25. [Google Scholar] [CrossRef]
- Sekar, V.; Anandasadagopan, S.K.; Ganapasam, S. Genistein regulates tumor microenvironment and exhibits anticancer effect in dimethyl hydrazine-induced experimental colon carcinogenesis. Biofactors 2016, 42, 623–637. [Google Scholar] [CrossRef]
- Havermann, S.; Chovolou, Y.; Humpf, H.U.; Watjen, W. Modulation of the Nrf2 signalling pathway in Hct116 colon carcinoma cells by baicalein and its methylated derivative negletein. Pharm. Biol. 2016, 54, 1491–1502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phan, T.; Nguyen, V.H.; A’Lincourt Salazar, M.; Wong, P.; Diamond, D.J.; Yim, J.H.; Melstrom, L.G. Inhibition of Autophagy Amplifies Baicalein-Induced Apoptosis in Human Colorectal Cancer. Mol. Oncolytics. 2020, 19, 1–7. [Google Scholar] [CrossRef]
- Yao, J.; Zhao, L.; Zhao, Q.; Zhao, Y.; Sun, Y.; Zhang, Y.; Miao, H.; You, Q.; Hu, R.; Guo, Q. NF-κ B and Nrf2 signaling pathways contribute to wogonin-mediated inhibition of inflammation-associated colorectal carcinogenesis. Cell Death Dis. 2014, 5, e1283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yao, J.; Hu, R.; Sun, J.; Lin, B.; Zhao, L.; Sha, Y.; Zhu, B.; You, Q.D.; Yan, T.; Guo, Q.L. Oroxylin a prevents inflammation-related tumor through down-regulation of inflammatory gene expression by inhibiting NF-κB signaling. Mol. Carcinog. 2014, 53, 145–158. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.; Chen, N.; Yao, J.; Zhao, Q.; Zhang, F.; Li, Z.Y.; You, Q.D.; Guo, Q.L. The role of Nrf2 and apoptotic signaling pathways in oroxylin A-mediated responses in HCT-116 colorectal adenocarcinoma cells and xenograft tumors. Anticancer Drugs 2012, 23, 651–658. [Google Scholar] [CrossRef]
- Bi, W.; He, C.N.; Li, X.X.; Zhou, L.Y.; Liu, R.J.; Zhang, S.; Li, G.Q.; Chen, Z.C.; Zhang, P.F. Ginnalin A from Kujin tea (Acer tataricum subsp. ginnala) exhibits a colorectal cancer chemoprevention effect via activation of the Nrf2/HO-1 signaling pathway. Food Funct. 2018, 9, 2809–2819. [Google Scholar] [CrossRef] [PubMed]
- Bi, W.; Gao, Y.; Shen, J.; He, C.; Liu, H.; Peng, Y.; Zhang, C.; Xiao, P. Traditional uses, phytochemistry, and pharmacology of the genus Acer (maple): A review. J. Ethnopharmacol. 2016, 189, 31–60. [Google Scholar] [CrossRef]
- Ma, H.; Wang, L.; Niesen, D.B.; Cai, A.; Cho, B.P.; Tan, W.; Gu, Q.; Xu, J.; Seeram, N.P. Structure activity related, mechanistic, and modeling studies of gallotannins containing a glucitol-core and α-glucosidase. RSC Adv. 2015, 5, 107904–107915. [Google Scholar] [CrossRef] [PubMed]
- Carroll, R.E.; Benya, R.V.; Turgeon, D.K.; Vareed, S.; Neuman, M.; Rodriguez, L.; Kakarala, M.; Carpenter, P.M.; McLaren, C.; Meyskens, F.L. Phase IIa clinical trial of curcumin for the prevention of colorectal neoplasia. Cancer Prev. Res. 2011, 4, 354–364. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, A.V.; Martinez, M.; Stamos, M.J.; Moyer, M.P.; Planutis, K.; Hope, C.; Holcombe, R.F. Results of a phase I pilot clinical trial examining the effect of plant-derived resveratrol and grape powder on Wnt pathway target gene expression in colonic mucosa and colon cancer. Cancer Manag. Res. 2009, 1, 25. [Google Scholar] [PubMed]
- Pintova, S.; Dharmupari, S.; Moshier, E.; Zubizarreta, N.; Ang, C.; Holcombe, R.F. Genistein combined with FOLFOX or FOLFOX–Bevacizumab for the treatment of metastatic colorectal cancer: Phase I/II pilot study. Cancer Chemother. Pharmacol. 2019, 84, 591–598. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, D.-Y.; Song, M.-Y.; Kim, E.-H. Role of Oxidative Stress and Nrf2/KEAP1 Signaling in Colorectal Cancer: Mechanisms and Therapeutic Perspectives with Phytochemicals. Antioxidants 2021, 10, 743. https://doi.org/10.3390/antiox10050743
Lee D-Y, Song M-Y, Kim E-H. Role of Oxidative Stress and Nrf2/KEAP1 Signaling in Colorectal Cancer: Mechanisms and Therapeutic Perspectives with Phytochemicals. Antioxidants. 2021; 10(5):743. https://doi.org/10.3390/antiox10050743
Chicago/Turabian StyleLee, Da-Young, Moon-Young Song, and Eun-Hee Kim. 2021. "Role of Oxidative Stress and Nrf2/KEAP1 Signaling in Colorectal Cancer: Mechanisms and Therapeutic Perspectives with Phytochemicals" Antioxidants 10, no. 5: 743. https://doi.org/10.3390/antiox10050743