Effects of Clostridium butyricum and a Bacteriophage Cocktail on Growth Performance, Serum Biochemistry, Digestive Enzyme Activities, Intestinal Morphology, Immune Responses, and the Intestinal Microbiota in Rabbits
Abstract
:1. Introduction
2. Results
2.1. Growth Performance
2.2. Serum Biochemical Index Analysis
2.3. Digestive Enzyme Activity
2.4. Antioxidant Status of Small Intestinal Tissues
2.5. Morphological Assessment of the Small Intestinal Mucosa
2.6. Immune Responses
2.7. Tight Junction Protein mRNA Level
2.8. The Effect of Treatment on Cecal Microbial Diversity
3. Discussion
4. Materials and Methods
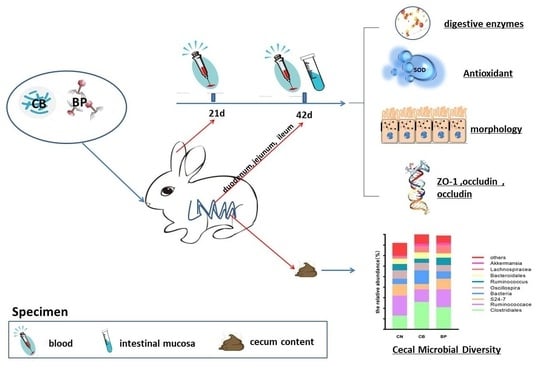
4.1. Animals, Treatment, and Designation
4.2. Experimental Design
4.3. Sample Collection
4.4. Growth Performance Evaluation
4.5. Serum Biochemical and Immune Index Analysis
4.6. Digestive Enzyme Activity and Antioxidant Indexes
4.7. Morphological Measurement of the Small Intestinal Mucosa
4.8. Secretory Immunoglobulin A
4.9. Quantitative PCR Analysis of Gene Expression
4.10. High-Throughput Sequencing of the Cecum Microbiota
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Zhu, Y.; Wang, C.; Li, F. Impact of dietary fiber/starch ratio in shaping caecal microbiota in rabbits. Can. J. Microbiol. 2015, 61, 771–784. [Google Scholar] [CrossRef] [Green Version]
- Mary, B.D. Normal bacterial flora of the rabbit gastrointestinal tract: A clinical approach. Nutr. Res. Rev. 2000, 13, 279–299. [Google Scholar]
- Bacanlı, M.; Başaran, N. Importance of antibiotic residues in animal food. Food Chem. Toxicol. 2019, 125, 462–466. [Google Scholar] [CrossRef]
- Erik, K.; Knudsen, K.E.B. Development of antibiotic resistance and options to replace antimicrobials in animal diets. Proc. Nutr. Soc. 2001, 60, 291–299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, G.; Hao, H.; Xie, S.; Wang, X.; Dai, M.; Huang, L.; Yuan, Z. Antibiotic alternatives: The substitution of antibiotics in animal husbandry? Front. Microbiol. 2014, 5, 217. [Google Scholar] [CrossRef] [Green Version]
- El-Deep, M.H.; Amber, K.A.; Eid, Y.Z.; Alrashood, S.T.; Khan, H.A.; Sakr, M.S.; Dawood, M.A.O. The influence of dietary chicken egg lysozyme on the growth performance, blood health, and resistance against escherichia coli in the growing rabbits’ cecum. Front. Vet. Sci. 2020, 7, 579576. [Google Scholar] [CrossRef] [PubMed]
- Ibtisham, F.; Nawab, A.; Niu, Y.; Wang, Z.; Wu, J.; Xiao, M.; An, L. The effect of ginger powder and Chinese herbal medicine on production performance, serum metabolites and antioxidant status of laying hens under heat-stress condition. J. Therm. Biol. 2019, 81, 20–24. [Google Scholar] [CrossRef]
- Yu, Q.; Yuan, L.; Deng, J.; Yang, Q. Lactobacillus protects the integrity of intestinal epithelial barrier damaged by pathogenic bacteria. Front. Cell. Infect. Microbiol. 2015, 5, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Chen, X.; Liu, P.; Zhao, J.; Sun, J.; Guan, W.; Johnston, L.J.; Levesque, C.L.; Fan, P.; He, T.; et al. Dietary clostridium butyricum induces a phased shift in fecal microbiota structure and increases the acetic acid-producing bacteria in a weaned piglet model. J. Agric. Food Chem. 2018, 66, 5157–5166. [Google Scholar] [CrossRef]
- Li, H.-H.; Li, Y.-P.; Zhu, Q.; Qiao, J.-Y.; Wang, W.-J. Dietary supplementation with Clostridium butyricum helps to improve the intestinal barrier function of weaned piglets challenged with enterotoxigenic Escherichia coli K88. J. Appl. Microbiol. 2018, 125, 964–975. [Google Scholar] [CrossRef]
- Zhang, L.; Cao, G.T.; Zeng, X.F.; Zhou, L.; Ferket, P.R.; Xiao, Y.P.; Chen, A.G.; Yang, C.M. Effects of Clostridium butyricum on growth performance, immune function, and cecal microbiota in broiler chickens challenged with Escherichia coli K88. Poult. Sci. 2014, 93, 46–53. [Google Scholar] [CrossRef]
- Sharma, S.; Chatterjee, S.; Datta, S.; Prasad, R.; Dubey, D.; Prasad, R.K.; Vairale, M.G. Bacteriophages and its applications: An overview. Folia Microbiol. 2016, 62, 17–55. [Google Scholar] [CrossRef] [PubMed]
- Haq, I.U.; Chaudhry, W.N.; Akhtar, M.N.; Andleeb, S.; Qadri, I. Bacteriophages and their implications on future biotechnology: A review. Virol. J. 2012, 9, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bragg, R.; van der Westhuizen, W.; Lee, J.Y.; Coetsee, E.; Boucher, C. Bacteriophages as potential treatment option for antibiotic resistant bacteria. Adv. Exp. Med. Biol. 2014, 807, 97–110. [Google Scholar] [PubMed]
- Cao, G.; Tao, F.; Hu, Y.; Li, Z.; Zhang, Y.; Deng, B.; Zhan, X. Positive effects of a Clostridium butyricum-based compound probiotic on growth performance, immune responses, intestinal morphology, hypothalamic neurotransmitters, and colonic microbiota in weaned piglets. Food Funct. 2019, 10, 2926–2934. [Google Scholar] [CrossRef]
- Wittebole, X.; de Roock, S.; Opal, S.M. A historical overview of bacteriophage therapy as an alternative to antibiotics for the treatment of bacterial pathogens. Virulence 2014, 5, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Wang, F.; Ling, Z.; Yu, X.; Chen, W.; Li, H.; Jin, J.; Pang, M.; Zhang, H.; Yu, J.; et al. Clostridium butyricum attenuates cerebral ischemia/reperfusion injury in diabetic mice via modulation of gut microbiota. Brain Res. 2016, 1642, 180–188. [Google Scholar] [CrossRef]
- Corrêa-Oliveira, R.; Fachi, J.L.; Vieira, A.; Sato, F.T.; Vinolo, M.A.R. Regulation of immune cell function by short-chain fatty acids. Clin. Transl. Immunol. 2016, 5, e73. [Google Scholar] [CrossRef]
- Takahashi, M.; McCartney, E.; Knox, A.; Francesch, M.; Oka, K.; Wada, K.; Ideno, M.; Uno, K.; Kozłowski, K.; Jankowski, J.; et al. Effects of the butyric acid-producing strain Clostridium butyricum MIYAIRI 588 on broiler and piglet zootechnical performance and prevention of necrotic enteritis. Anim. Sci. J. 2018, 89, 895–905. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Latif, M.A.; Swelum, A.A.; Swelum, A.A.; Saadeldin, I.M.; Elbestawy, A.R.; Shewita, R.S.; Ba-Awadh, H.A.; Alowaimer, A.N.; El-Hamid, H.S.A. Single and combined effects of Clostridium butyricum and Saccharomyces cerevisiae on growth indices, intestinal health, and immunity of broilers. Animals 2018, 8, 184. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Kim, J.; Shin, H.; Kim, M.; Lee, J.; Kim, G.-B.; Kil, D. Effect of dietary supplementation of bacteriophage on performance, egg quality and caecal bacterial populations in laying hens. Br. Poult. Sci. 2015, 56, 132–136. [Google Scholar] [CrossRef] [PubMed]
- Gebru, E.; Lee, J.S.; Son, J.C.; Yang, S.Y.; Shin, S.A.; Kim, B.; Kim, M.K.; Park, S.C. Effect of probiotic-, bacteriophage-, or organic acid-supplemented feeds or fermented soybean meal on the growth performance, acute-phase response, and bacterial shedding of grower pigs challenged with Salmonella enterica serotype Typhimurium. J. Anim. Sci. 2010, 88, 3880–3886. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Wang, Z.; Zou, T.; Chen, J.; Li, G.; Zheng, L.; Li, S.; You, J. Bacteriophage as an Alternative to antibiotics promotes growth performance by regulating intestinal inflammation, intestinal barrier function and gut microbiota in weaned piglets. Front. Vet. Sci. 2021, 8, 623899. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, B.; Lee, H.S.; Bae, E.A.; Lee, H.; Ahn, Y.T.; Lim, K.S.; Huh, C.S.; Kim, D.H. Lactobacillus suntoryeus inhibits pro-inflammatory cytokine expression and TLR-4-linked NF-κB activation in experimental colitis. Int. J. Colorectal Dis. 2009, 24, 231–237. [Google Scholar] [CrossRef]
- Ghasemi, H.A.; Shivazad, M.; Mirzapour Rezaei, S.S.; Karimi Orshizi, M.A. Effect of synbiotic supplementation and dietary fat sources on broiler performance, serum lipids, muscle fatty acid profile and meat quality. Br. Poult. Sci. 2016, 57, 71–83. [Google Scholar] [CrossRef]
- Switzar, L.; Giera, M.; Niessen, W.M.A. Protein digestion: An overview of the available techniques and recent developments. J. Proteome Res. 2013, 12, 1067–1077. [Google Scholar] [CrossRef]
- Wang, J.; Ni, X.; Wen, B.; Zhou, Y.; Liu, L.; Zeng, Y.; Zhao, W.; Khalique, A.; Wang, P.; Pan, K.; et al. Bacillus strains improve growth performance via enhancing digestive function and anti-disease ability in young and weaning rex rabbits. Appl. Microbiol. Biotechnol. 2020, 104, 4493–4504. [Google Scholar] [CrossRef]
- Gostner, J.; Becker, K.; Fuchs, D.; Sucher, R. Redox regulation of the immune response. Redox Rep. 2013, 18, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Muri, J.; Kopf, M. Redox regulation of immunometabolism. Nat. Rev. Immunol. 2021, 21, 363–381. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.B.; Du, W.; Fu, A.K.; Zhang, X.P.; Huang, Y.; Lee, K.H.; Yu, K.; Li, W.F.; Li, Y.L. Intestinal microbiota and oral administration of Enterococcus faecium associated with the growth performance of new-born piglets. Benef. Microbes 2016, 7, 529–538. [Google Scholar] [CrossRef]
- Long, L.; Kang, B.; Jiang, Q.; Chen, J. Effects of dietary Lycium barbarum polysaccharides on growth performance, digestive enzyme activities, antioxidant status, and immunity of broiler chickens. Poult. Sci. 2020, 99, 744–751. [Google Scholar] [CrossRef]
- Liao, X.D.; Ma, G.; Cai, J.; Fu, Y.; Yan, X.Y.; Wei, X.B.; Zhang, R.J. Effects of Clostridium butyricum on growth performance, antioxidation, and immune function of broilers. Poult. Sci. 2015, 94, 662–667. [Google Scholar] [CrossRef]
- Jahns, F.; Wilhelm, A.; Jablonowski, N.; Mothes, H.; Greulich, K.O.; Glei, M. Butyrate modulates antioxidant enzyme ex-pression in malignant and non-malignant human colon tissues. Mol. Carcinog. 2015, 54, 249–260. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.; Wu, R.; Ma, G.; Zhao, L.; Zheng, Z.; Zhang, R. Effects of Clostridium butyricum on antioxidant properties, meat quality and fatty acid composition of broiler birds. Lipids Health Dis. 2015, 14, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Przerwa, A.; Zimecki, M.; Świtała-Jeleń, K.; Dąbrowska, K.; Krawczyk, E.; Łuczak, M.; Weber-Dąbrowska, B.; Syper, D.; Międzybrodzki, R.; Górski, A. Effects of bacteriophages on free radical production and phagocytic functions. Med Microbiol. Immunol. 2006, 195, 143–150. [Google Scholar] [CrossRef]
- Chen, Y.; Cheng, Y.; Wen, C.; Kang, Y.; Wang, A.; Zhou, Y. Effects of dietary synbiotic supplementation as an alternative to antibiotic on the growth performance, carcass characteristics, meat quality, immunity, and oxidative status of cherry valley ducks. J. Poult. Sci. 2018, 55, 182–189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, C.M.; Cao, G.T.; Ferket, P.; Liu, T.T.; Zhou, L.; Zhang, L.; Xiao, Y.P.; Chen, A.G. Effects of probiotic, Clostridium butyricum, on growth performance, immune function, and cecal microflora in broiler chickens. Poult. Sci. 2012, 91, 2121–2129. [Google Scholar] [CrossRef]
- Swaggerty, C.L.; Kogut, M.H.; Ferro, P.J.; Rothwell, L.; Pevzner, I.Y.; Kaiser, P. Differential cytokine mRNA expression in heterophils isolated from Salmonella-resistant and -susceptible chickens. Immunology 2004, 113, 139–148. [Google Scholar] [CrossRef]
- Ulluwishewa, D.; Anderson, R.C.; McNabb, W.C.; Moughan, P.J.; Wells, J.M.; Roy, N.C. Regulation of tight junction permeability by intestinal bacteria and dietary components. J. Nutr. 2011, 141, 769–776. [Google Scholar] [CrossRef] [Green Version]
- Mantis, N.J.; Rol, N.; Corthésy, B. Secretory IgA’s complex roles in immunity and mucosal homeostasis in the gut. Mucosal Immunol. 2011, 4, 603–611. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Hou, S.; Peng, W.; Lin, Q.; Chen, F.; Yang, L.; Li, F.; Huang, X. Oral administration of Lactobacillus delbrueckii during the suckling phase improves antioxidant activities and immune responses after the weaning event in a piglet model. Oxidative Med. Cell. Longev. 2019, 2019, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bai, K.; Feng, C.; Jiang, L.; Zhang, L.; Zhang, J.; Zhang, L.; Wang, T. Dietary effects of Bacillus subtilis fmbj on growth per-formance, small intestinal morphology, and its antioxidant capacity of broilers. Poult. Sci. 2018, 97, 2312–2321. [Google Scholar] [CrossRef] [PubMed]
- Long, M.; Yang, S.; Li, P.; Song, X.; Pan, J.; He, J.; Zhang, Y.; Wu, R. Combined use of C. butyricum Sx-01 and L. salivarius C-1-3 improves intestinal health and reduces the amount of lipids in serum via modulation of gut microbiota in mice. Nutrients 2018, 10, 810. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Li, S.; Zheng, J.; Li, W.; Jiang, X.; Zhao, X.; Li, J.; Che, L.; Lin, Y.; Xu, S.; et al. Effects of dietary Clostridium butyricum supplementation on growth performance, intestinal development, and immune response of weaned piglets challenged with lipopolysaccharide. J. Anim. Sci. Biotechnol. 2018, 9, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Hosseindoust, A.R.; Lee, S.H.; Kim, J.S.; Choi, Y.H.; Kwon, I.K.; Chae, B.J. Productive performance of weanling piglets was improved by administration of a mixture of bacteriophages, targeted to control Coliforms and Clostridium spp. shedding in a challenging environment. J. Anim. Physiol. Anim. Nutr. 2016, 101, e98–e107. [Google Scholar] [CrossRef]
- Fernandes, J.; Su, W.; Rahat-Rozenbloom, S.; Wolever, T.M.; Comelli, E.M. Adiposity, gut microbiota and faecal short chain fatty acids are linked in adult humans. Nutr. Diabetes 2014, 4, e121. [Google Scholar] [CrossRef]
- Everard, A.; Belzer, C.; Geurts, L.; Ouwerkerk, J.P.; Druart, C.; Bindels, L.B.; Guiot, Y.; Derrien, M.; Muccioli, G.G.; Delzenne, N.M.; et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc. Natl. Acad. Sci. USA 2013, 110, 9066–9071. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dao, M.C.; Everard, A.; Aron-Wisnewsky, J.; Sokolovska, N.; Prifti, E.; Verger, E.O.; Kayser, B.D.; Levenez, F.; Chilloux, J.; Hoyles, L.; et al. Akkermansia muciniphila and improved metabolic health during a dietary intervention in obesity: Relationship with gut microbiome richness and ecology. Gut 2016, 65, 426–436. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.; Cao, G.; Zhang, H.; Li, Q.; Yang, C. Effects of Clostridium butyricum and Enterococcus faecalis on growth performance, immune function, intestinal morphology, volatile fatty acids, and intestinal flora in a piglet model. Food Funct. 2019, 10, 7844–7854. [Google Scholar] [CrossRef]
- Ridaura, V.K.; Faith, J.J.; Rey, F.E.; Cheng, J.; Duncan, A.E.; Kau, A.; Griffin, N.W.; Lombard, V.; Henrissat, B.; Bain, J.R.; et al. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science 2013, 341, 1241214. [Google Scholar] [CrossRef] [Green Version]
- Scanlan, P.D. Bacteria–bacteriophage coevolution in the human gut: Implications for microbial diversity and functionality. Trends Microbiol. 2017, 25, 614–623. [Google Scholar] [CrossRef] [PubMed]
- Bakhshinejad, B.; Ghiasvand, S. Bacteriophages in the human gut: Our fellow travelers throughout life and potential bi-omarkers of heath or disease. Virus Res. 2017, 240, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Barr, J.J.; Auro, R.; Furlan, M.; Whiteson, K.L.; Erb, M.L.; Pogliano, J.; Stotland, A.; Wolkowicz, R.; Cutting, A.S.; Doran, K.S.; et al. Bacteriophage adhering to mucus provide a non-host-derived immunity. Proc. Natl. Acad. Sci. USA 2013, 110, 10771–10776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]









| Parameter | CN | CB | BP | p-Value |
|---|---|---|---|---|
| (5 weeks old) Initial BW (g) | 682.8 ± 10.03 | 690.4 ± 52.17 | 692.1 ± 45.72 | 0.942 |
| (11 weeks old) Final BW (g) | 1215 ± 30.38 b | 1354 ± 41.17.1 a | 1351 ± 34.84 a | 0.0179 |
| ADG (g) | 12.62 ± 1.41 b | 15.80 ± 2.05 a | 15.68 ± 1.28 a | 0.0273 |
| ADFI (g) | 105.24 ± 3.24 b | 109.83 ± 3.32 a | 108.73 ± 3.62 ab | 0.0484 |
| F/G | 8.41 ± 1.61 b | 6.95 ± 1.93 a | 6.93 ± 1.06 a | 0.0379 |
| Diet Composition | Percentage (%) | Components | Nutrient Levels |
|---|---|---|---|
| Corn | 14 | Total energy (kCAL.kg−1) | 3826.88 |
| Bran | 18 | Crude protein (%) | 13.42 |
| Bean cake | 8 | Calcium (%) | 1.43 |
| Grass powder | 30.67 | Total phosphorus (%) | 0.51 |
| Malt root | 10 | Lysine (%) | 0.719 |
| Big bran | 16 | Methionine (%) | 0.234 |
| Mountain flour | 0.83 | Cystine (%) | 0.268 |
| Salt | 0.5 | ||
| Premix a | 2 |
| * Gene | Prime Sequence (5′→3′) | Size (bp) | Accession |
|---|---|---|---|
| ZO-1 | F: CCTGCGAAACCCACCAAA | 293 | XM_008269782.1 |
| R: ATGCTGTCGAAAGGTCAGGG | |||
| Claudin | F: GCAAGAGGCCGTATCCAGAG | 193 | NM_001089316.1 |
| R: AGTCCGTCTCGTAGTGGTCT | |||
| Occludin | F: GGAGCAAAAGATGCGCATGG | 207 | XM_008262320.1 |
| R: AATTGACAGGGGTCAAAGGGT | |||
| GAPDH | F: TGTTTGTGATGGGCGTGAA | 129 | NC_013676.1 |
| R: CCTCCACAATGCCGAAGT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, P.; Cui, X.; Wang, Z.; Xiao, C.; Ji, Q.; Wei, Q.; Huang, Y.; Bao, G.; Liu, Y. Effects of Clostridium butyricum and a Bacteriophage Cocktail on Growth Performance, Serum Biochemistry, Digestive Enzyme Activities, Intestinal Morphology, Immune Responses, and the Intestinal Microbiota in Rabbits. Antibiotics 2021, 10, 1347. https://doi.org/10.3390/antibiotics10111347
Huang P, Cui X, Wang Z, Xiao C, Ji Q, Wei Q, Huang Y, Bao G, Liu Y. Effects of Clostridium butyricum and a Bacteriophage Cocktail on Growth Performance, Serum Biochemistry, Digestive Enzyme Activities, Intestinal Morphology, Immune Responses, and the Intestinal Microbiota in Rabbits. Antibiotics. 2021; 10(11):1347. https://doi.org/10.3390/antibiotics10111347
Chicago/Turabian StyleHuang, Pan, Xuemei Cui, Zhipeng Wang, Chenwen Xiao, Quanan Ji, Qiang Wei, Yee Huang, Guolian Bao, and Yan Liu. 2021. "Effects of Clostridium butyricum and a Bacteriophage Cocktail on Growth Performance, Serum Biochemistry, Digestive Enzyme Activities, Intestinal Morphology, Immune Responses, and the Intestinal Microbiota in Rabbits" Antibiotics 10, no. 11: 1347. https://doi.org/10.3390/antibiotics10111347