Antibacterial Potential of Caesalpinia coriaria (Jacq) Willd Fruit against Aeromonas spp. of Aquaculture Importance
Abstract
:Simple Summary
Abstract
1. Introduction
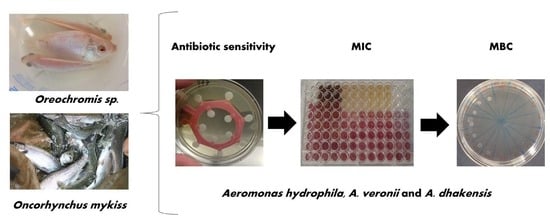
2. Materials and Methods
2.1. Plant Material
2.2. Hydroalcoholic Extract
2.3. Hydroalcoholic Extract Bipartition
2.4. Gallic Acid
2.5. Bacteria and Culture Conditions
2.6. Antimicrobial Sensitivity Testing
2.7. Antibacterial Activity
2.8. Bactericidal and Bacteriostatic Effect
2.9. Cytotoxicity Test with Artemia salina
2.10. Statistical Analysis
3. Results
3.1. Antimicrobial Sensitivity
3.2. Antibacterial Activity
3.3. Bacteriostatic and Bactericidal Activity
3.4. Cytotoxicity of Extract, Fractions, and Gallic Acid against Artemia salina
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. El Estado Mundial de la Pesca y La Acuicultura 2020. La Sostenibilidad en Acción; FAO: Roma, Italy, 2020. [Google Scholar] [CrossRef]
- CONAPESCA. Anuario Estadístico de Acuacultura y Pesca. 2018. Available online: https://www.conapesca.gob.mx/work/sites/cona/dgppe/2018/ANUARIO_2018.pdf (accessed on 6 December 2021).
- Bao, L.; Chen, Y.; Li, H.; Zhang, J.; Wu, P.; Ye, K.; Ai, H.; Chu, W. Dietary Ginkgo biloba leaf extract alters immune-related gene expression and disease resistance to Aeromonas hydrophila in common carp Cyprinus carpio. Fish Shellfish Immunol. 2019, 94, 810–818. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Latif, H.M.R.; Dawood, M.A.O.; Menanteau-Ledouble, S.; El-Matbouli, M. The nature and consequences of co-infections in tilapia: A review. J. Fish Dis. 2020, 43, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Bandeira, J.G.; Souza, C.F.; Dellaméa, B.M.; Nunes, D.S.; Petri, S.B.; Tasca, C.; Veras, M.R.H.; Palmira, C.V.A.; Baldisserotto, B. Plant essential oils against bacteria isolated from fish: An in vitro screening and in vivo efficacy of Lippia origanoides. Cienc. Rural. 2019, 49. [Google Scholar] [CrossRef]
- Reverter, M.; Botemps, N.; Lecchini, D.; Banaigs, B.; Sasal, P. Use of plant extracts in fish aquaculture as an alternative to chemotherapy: Corrent status and future perspectives. Aquaculture 2014, 433, 50–61. [Google Scholar] [CrossRef]
- Garcia-Valenzuela, M.H.; Orozco-Medina, C.; Molina-Maldonado, C. Efecto antibacteriano del aceite esencial de orégano (Lipia berlandieri) en bacterias patógenas de camarón Litopenaeus vannamei. Hidrobiológica 2012, 22, 201–206. Available online: http://www.scielo.org.mx/scielo.php?script=sci_arttext&pid=S0188-88972012000300002&lng=es&tlng=es (accessed on 6 December 2021).
- Culot, A.; Grosset, N.; Gautier, M. Overcoming the challenges of phage therapy for industrial aquaculture: A review. Aqua-culture 2019, 513, 734423. [Google Scholar] [CrossRef] [Green Version]
- Starliper, C.E.; Ketola, H.G.; Noyes, A.D.; Schill, W.B.; Henson, F.G.; Chalupnicki, M.A.; Dittman, D.E. An investigation of the bactericidas activity of selected essential oils to Aeromonas spp. J. Adv. Res. 2014, 6, 89. [Google Scholar] [CrossRef] [Green Version]
- Martin-Carnahan, A.; Joseph, S.W. Aeromonas. Bergey’s Manual of Systematics of Archaea and Bacteria; Jhon Wiley and Sons: Hoboken, NJ, USA, 2015; pp. 1–44. [Google Scholar] [CrossRef]
- Constantino, C.F.; Armijo, O.A.; Osorio, S.D.; Chávez, S.L. Infección por Aeromonas hydrophila e Ichthyophthirius multifiliis en trucha (Oncorhynchus mykiss, Walbaum) y tilapia (Oreochromis aureus, L) de un centro de acopio de Morelos, México. Estudio patológico. Vet. Mex. 1997, 28, 59–62. Available online: https://www.medigraphic.com/pdfs/vetmex/vm-1997/vm971l.pdf (accessed on 6 December 2021).
- Eissa, I.A.M.; El-Lamei, M.; Sherif, M.; Desuky, E.; Zaki, M.; Bakry, M. Aeromonas veronii biovar sobria a causative agent of mass mortalities in cultured Nile tilapia in El-Sharkia governorate. Egypt. Life Sci. J. 2015, 12, 90–97. Available online: http://www.lifesciencesite.com/lsj/life120515/011_28610life120515_90_97.pdf (accessed on 6 December 2021).
- Esteve, C.; Alcaide, E.; Blasco, M.D. Aeromonas hydrophila subsp. dhakensis isolated from feces, water and fish in mediterranean spain. Microbes Environ. 2012, 27, 367–373. [Google Scholar] [CrossRef] [Green Version]
- Soto-Rodríguez, S.A.; Lozano-Olvera, R.; García-Gasca, M.T.; Abad-Rosales, S.M.; Gómez-Gil, B.; Ayala-Arellano, J. Virulence of the fish pathogen Aeromonas dhakensis genes involved, characterization and histopathology of experimentally infected hybrid tilapia. Dis. Aquat. Org. 2018, 129, 107–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, W.; Zhou, S.; Chu, W. Comparative proteomic analisis of sensitive and multidrog resistant Aeromonas hydrophila isolated from deseased fish. Microb. Pathog. 2019, 139, 103930. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.-H.; Wu, Z.-H.; Jian, J.-C.; Lu, Y.-S.; Tang, J.-F. Characterization of pathogenic Aeromonas veronii bv. Veronii associated with ulcerative syndrome from Chinese longsnout catfish (Leiocassis longirostris Günther). Raz. J. Microbiol. 2011, 43, 382–388. [Google Scholar] [CrossRef] [Green Version]
- Van Hai, N. The use of medicinal plants as immunostimulants in aquaculture: A review. Aquaculture 2015, 446, 88–96. [Google Scholar] [CrossRef]
- Harikrishnan, R.; Balasundaram, C.; Heo, M. Impacto of plant products on innate and adaptive immune system of cultured finfish and shellfish. Aquaculture 2011, 317, 1–15. [Google Scholar] [CrossRef]
- Rangel-López, L.; Zaragoza-Bastida, A.; Valladares-Carranza, B.; Peláez-Acero, A.; Sosa-Gutiérrez, C.G.; Hetta, H.F.; Batiha, G.E.; Alqahtani, A.; Rivero-Perez, N. In vitro antibacterial potential of Salix babylonica extract against bacteria that affect Oncorhynchus mykiss and Oreochromis spp. Animals 2020, 10, 1340. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.W.; Sim, K.Y.; Wendy, W.; Zulhisyam, A.K. Peperomia pellucida leaf extract as immunostimulator in controlling motile aeromonad septicemia due to Aeromonas hydrophila in red hibrid tilapia, Oreochromis spp. Farm. Vet. World. 2016, 9, 231–234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taynapun, K.U.; Mueangkan, N.; Chirapongsatonkul, N. Efficacy of herbal extracts to control multi-antibiotics resistant (MAR) Aeromonas veronii isolated from motile Aeromonas septicemia (MAS)-exhibiting Nile tilapia (Oreochromis niloticus). Int. J. Agric. Technol. 2018, 14, 2191–2206. Available online: www.ijat-aatsea.com/pdf/v14_n7_2018_%IJAT_14(7)_2018_U-taynapun,%20K..pdf (accessed on 10 December 2021).
- Olmedo-Juárez, A.; Briones-Robles, T.I.; Zaragoza-Bastida, A.; Zamilpa, A.; Ojeda-Ramírez, D.; Mendoza de Gives, P.; Olivares-Pérez, J.; Rivero-Perez, N. Antibacterial activity of compounds isolated from Caesalpinia coriaria (Jacq) willd against important bacteria in public health. Microb. Pathog. 2019, 136, 103660. [Google Scholar] [CrossRef]
- Mohana, D.C.; Raveesha, K.A. Anti-bacterial activity of Caesalpinia coriaria (Jacq.) Willd. against plant pathogenic Xan-thomonas pathovars: An eco-friendly approach. J. Agric. Sci. Technol. 2006, 2, 317–327. [Google Scholar]
- García-Hernández, C.; Rojo-Rubio, R.; Olmedo-Juárez, A.; Zamilpa, A.; Mendoza de Gives, P.; Antonio-Romo, I.A.; Aguilar-Marcelino, L.; Arece-García, J.; Tapia-Maruri, D. Galloyl derivatives from Caesalpinia coriaria exhibit in vitro ovicidal activity against cattle gastrointestinal parasitic nematodes. Exp. Parasitol. 2019, 200, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Rojas, J.; Velasco, J.; Buitrago, A.; Mender, T.; Rojas, J. Evaluación de la actividad antimicrobiana de plantas medicinales seleccionadas del jardín botánico del Orinoco, municipio Heres, Estado Bolivar. Rev. Fac. Farm. 2016, 58, 2–10. [Google Scholar]
- Jeeva, K.; Thiyagarajan, M.; Elangovan, V.; Geetha, N.; Venkatachalam, P. Caesalpinia coriaria leaf extracts mediated biosyn-thesis of metallic silver nanoparticles and their antibacterial activity against clinically isolated pathogens. Ind. Crops Prod. 2014, 52, 714–720. [Google Scholar] [CrossRef]
- Cruz, M.C. Pruebas de sensibilidad y resistencia bacteriana frente a diferentes concentraciones de extracto de Caesalpinia coriaria (Guatanamá). Cienc. Soc. 2007, 32, 8–20. Available online: https://www.redalyc.org/articulo.oa?id=870/87032107 (accessed on 1 December 2021). [CrossRef]
- Clinical and Laboratory Standards Institute. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically (Approved Standard); CLSI document M7-A5; CLSI: Wayne, PA, USA, 2012. [Google Scholar]
- Zaragoza-Bastida, A.; Flores-Aguilar, S.C.; Aguilar-Castro, L.M.; Morales-Ubaldo, A.L.; Valladares-Carranza, B.; Rangel-López, L.; Olmedo-Juárez, A.; Rosenfeld-Miranda, C.E.C.; Rivero-Pérez, N. Antibacterial and hemolytic activity of Crotalus triseriatus and Crotalus ravus Venom. Animals 2020, 10, 281. [Google Scholar] [CrossRef] [Green Version]
- Morales-Ubaldo, A.; Rivero-Pérez, N.; Avila-Ramos, F.; Aquino-Torres, E.; Prieto-Mendez, J.; Hetta, H.F.; El-Saber Batiha, G.; Zaragoza-Bastida, A. Bactericidal activity of Larrea tridentata hydroalcoholic extract against phytopathogenic bacteria. Agronomy 2021, 11, 975. [Google Scholar] [CrossRef]
- González-Alamilla, E.; Rivas-Jacobo, M.; Sosa-Gutiérrez, C.; Delgadillo-Ruiz, L.; Valladares-Carranza, B.; Rosenfeld-Miranada, C.; Zaragoza-Bastida, A.; Rivero-Pérez, N. Efecto antibacteriano del extracto metanolico de Salix babylonica sobre bacterias de importancia en salud pública. Abanico Vet. 2020, 10, 1–11. [Google Scholar] [CrossRef]
- Djihane, B.; Wafa, N.; Elkhamssa, S.; Haro, J.P.D.; Angeles, E.M.; Mouhamed, M.Z. Chemical constituents of Helichrysum italicum (Roth) G. Don essential oil and their antimicrobial activity against Gram-positive and Gram-negative bacteria, fila-mentous fungi and Candida albicans. Saudi Pharm. J. 2016, 25, 780–787. [Google Scholar] [CrossRef]
- Solis, P.N.; Wright, C.W.; Anderson, M.M.; Gupta, M.P.; Phillipson, D. A microwell cytotoxicity assay using Artemia salina (Brine shrimp). Planta Med. 1993, 59, 250–252. [Google Scholar] [CrossRef]
- Rivero-Perez, N.; Hernández-Alvarado, J.L.; Valladares-Carranza, B.; Delgadillo-Ruiz, L.; Ojeda-Ramírez, D.; Gutiérrez, C.G.S.; Morales-Ubaldo, A.L.; Vega-Sanchez, V.; Zaragoza-Bastida, A. Salix babylonica L. as a natural anticoccidial alter-native in growing rabbits. Evid.-Based Complement. Altern. Med. 2019, 2019, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Sulit, J.E.B.; Atienza, L.M. Cytotoxic effect of the methanolic extract of selected edible weeds on Artemia salina nauplii. J. Nutr. Res. Food Sci. 2020, 1, 4. Available online: https://cienciaworld.com/cytotoxic-effect-of-the-mettanolic-extract-of-selected-edible-weeds.pdf (accessed on 15 December 2021).
- Mentor, R.H.; Blagica, J.; Tatjana, K.P. Toxicological evaluation of the plant products using brine shrimp (Artemia salina L.) model. Maced. Pharm. Bull. 2014, 60, 9–18. Available online: Bulletin.mfd.org.mk/volumenes/Volume%2060/60_002.pdf (accessed on 20 December 2021).
- Bilen, S.; Elbeshti, H.T.A.G. A new potential therapeutic remedy against Aeromonas hydrophila infection in rainbow trout (Oncorhynchus mykiss) using tetra Cotinus coggygria. J. Fish Dis. 2019, 42, 1369–1381. [Google Scholar] [CrossRef] [PubMed]
- Stratev, D.; Odeyemi, O.A. Antimicrobial resistance of Aeromonas hydrophila isolated from different food sources: A mini-review. J. Infect. Public Health. 2016, 9, 535–544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, J.H.; Han, J.J.; Kim, H.J.; Kang, S.G.; Park, S.W. Fist reporto Aeromonas veronii infection in farmed Israeli carp Cyprinus carpio in Korea. J. Fish Pathol. 2010, 23, 165–176. [Google Scholar]
- López-Pueyo, M.J.; Barcenilla-Gaite, F.; Amaya-Villar, R.; Garnacho-Montero, J. Multirresistencia antibiótica en unidades de críticos. Med. Intensiva. 2011, 35, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Hardi, E.H.; Kusuma, I.W.; Suwinarti, W.; Agustina, A.I.; Nugroho, R.A. Antibacterial activities of some borneo plant extracts against áthogenic bacteria of Aeromonas hydrophila and Pseudomonas sp. AACL Bioflux. 2017, 9, 638–646. [Google Scholar]
- Kavitha, M.; Raja, M.; Kamaraj, C.; Balasubramaniam, V.; Balasubramaniam, G.; Perumal, P. In vitro antimicrobial activity of Azadirachta indica (Leaves) against fish pathogenic bacteria isolated from naturally infected Dawkinsia filamentosa (Blacjspot barb). Med. Aromat. Plants. 2017, 6, 1–7. [Google Scholar] [CrossRef]
- Pachanawan, A.; Phumkhachorn, P.; Rattanachaikunsopon, P. Potential of Psidium guajava suplemented fish diets in controlling Aeromonas hydrophila infection in tilapia (Oreochromis niloticus). J. Biosci. Bioeng. 2008, 106, 419–424. [Google Scholar] [CrossRef] [Green Version]
- Prasad, V.G.N.V.; Krishna, B.V.; Swamy, P.L.; Rao, T.S.; Rao, G.S. Antibacterial synergy between quercetin and poly-phenolic acids against bacterial pathogens of fish. Asian Pac. J. Tropl. Dis. 2014, 4 (Suppl. 1), 326–329. [Google Scholar] [CrossRef]
- Chung, K.T.; Zha, G.; Stevens, E.; Simco, B.A.; Wei, C.I. Groeth inhibition of selected aquatic bacteria by taninic acid and related compounds. J. Aquat Anim Health 1995, 7, 46–49. [Google Scholar] [CrossRef]
- Kanchan, C.; Imjai, P.; Kanchan, N.; Panchai, K.; Hatai, K. Virulence of Aeromonas hydrophila in Siamese fighting fish (Betta splendens) and the bacterium susceptibility to some herbal plants. Iran. J. Fish. Sci. 2019, 18, 349–354. [Google Scholar] [CrossRef]
- Soberón, J.R.; Sgariglia, M.A.; Maderuelo, M.R.D.; Andina, M.L.; Sampietro, D.A.; Vattuone, M.A. Antibacterial activities of Ligaria cuneifolia and Jodina rhombifolia leaf extracts against phytopathogenic and clinical bacteria. J. Biosci. Bioeng. 2014, 118, 599–605. [Google Scholar] [CrossRef] [PubMed]
- Rivas-Cáceres, R.R.; Stephano-Hornedo, L.; Lugo, J.; Vaca, R.; Del Aguilla, P.; Yañez-Ocampo, G.; Mora-Herrera, M.E.; Camacho, D.L.M.; Cipriano-Salazar, M.; Adeniyi, A.P. Bactericidal effect of silver nanoparticles against the propagation of Clavibacter michiganensis infection in Lycopersicon esculentum Mill. Microb. Pathog. 2018, 115, 358–362. [Google Scholar] [CrossRef]
- Pizzani, P.; Matute, I.; De Martino, G.; Arias, A.; Godoy, S.; Pereira, L.; Palma, J.; Rengifo, M. Composición Fitoquímica y Nutricional de Algunos Frutos de Árboles de Interés Forrajero de Los Llanos Centrales de Venezuela. Rev. Cien. Fac. Vet. 2006, 47, 105–111. Available online: https://www.redalyc.org/articulo.oa?id=3731/373139066005 (accessed on 20 November 2021).
- Ávalos-Soto, J.; Treviño-Neávez, J.F.; Verde-Star, M.J.; Rivas-Morales, C.; Oranday-Cárdenas, A.; Moran-Martinez, J.; Serrano-Gallardo, L.B.; Morales-Rubio, M.E. Evaluación citotóxica de los extractos etanolicos de Azadirachta indica (A. Juss) sobre diferentes líneas celulares. Rev. Mex. Cienc. Farm. 2014, 45, 39–44. Available online: www.scielo.org.mx/pdf/rmcf/v45n3/v45n3a4.pdf. (accessed on 25 November 2021).
- Apu, A.S.; Muhit, M.A.; Tareq, S.M.; Pathan, A.H.; Jamaluddin, A.T.M.; Ahmed, M. Antimicrobial activity and brine shrimp lethality bioassay of the leaves extract of Dillenia indica Linn. J. Young Pharm. 2010, 2, 50–53. [Google Scholar] [CrossRef] [Green Version]

| Antibiotic | A. hydrophila | A. veronii | A. dhakensis |
|---|---|---|---|
| Cephalotin (30 µg) | 14(R) | 25(S) | 8(R) |
| Cefotaxime (30 µg) | 30(S) | 30(S) | 33(S) |
| Ciprofloxacin (5 µg) | 25(S) | 30(S) | 25(S) |
| Cloramphenicol (30 µg) | 25(S) | 30(S) | 30(S) |
| Nitrofurantoin (300 µg) | 25(S) | 22(S) | 25(S) |
| Ampicillin (10 µg) | 6(R) | 6(R) | 6(R) |
| Carbenicillina (100 µg) | 6(R) | 6(R) | 6(R) |
| Gentamicin (10 µg) | 20(S) | 15(S) | 20(S) |
| Netelmicin (30 µg) | 20(S) | 10(I) | 10(I) |
| Norfloxacin (10 µg) | 20(S) | 15(I) | 15(I) |
| Sulfamethoxazole/Trimethoprim (25 µg) | 22(S) | 12(S) | 20(S) |
| Amikacin (30 µg) | 20(S) | 6(R) | 6(R) |
| Treatment | Minimal Inhibitory Concentration (mg/mL) | ||
|---|---|---|---|
| A. hydrophila | A. veronii | A. dhakensis | |
| EHCc | 1.56 cB | 0.78 bA | 0.78 bA |
| Ac-FrEtCc | 0.09 aA | 0.78 bB | 0.78 bB |
| Ac-FrCc | 0.19 bA | 0.39 aB | 0.39 aC |
| Gallic acid | 0.09 aA | 3.12 cB | NA |
| P.C. (Kanamycin µg/mL) | 1 A | 4 C | 2 B |
| N.C. | NA | NA | NA |
| Valor de p | 0.0001 | 0.0001 | 0.0001 |
| Treatment | Minimal Bactericidal Concentration (mg/mL) | ||
|---|---|---|---|
| A. hydrophila | A. veronii | A. dhakensis | |
| HECc | 3.12 cB | 6.25 bC | 1.56 bA |
| Ac-FrEtCc | 0.19 bA | 6.25 bC | 3.12 cB |
| Ac-FrCc | 0.19 bA | 3.12 aA | 0.78 cB |
| Gallic acid | 0.09 aA | 6.25 bB | NA |
| P.C. (µg/mL) | 2 B | 16 C | 1 A |
| N.C. | NA | NA | NA |
| Valor de p | 0.0001 | 0.0001 | 0.0001 |
| Treatment | Ratio of MBC/MIC | ||
|---|---|---|---|
| A. hydrophila | A. veronii | A. dhakensis | |
| HECc | 2.0 (Bactericidal) | 8.0 (Bacteriostatic) | 2.0 (Bactericidal) |
| Ac-FrEtCc | 2.1 (Bactericidal) | 8.0 (Bacteriostatic) | 4.0 (Bactericidal) |
| Ac-FrCc | 1.0 (Bactericidal) | 8.9 (Bacteriostatic) | 2.0 (Bactericidal) |
| Gallic acid | 1.0 (Bactericidal) | 2.0 (Bactericidal) | NA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rangel-López, L.; Rivero-Perez, N.; Valladares-Carranza, B.; Olmedo-Juárez, A.; Delgadillo-Ruiz, L.; Vega-Sánchez, V.; Hori-Oshima, S.; Nassan, M.A.; Batiha, G.E.-S.; Zaragoza-Bastida, A. Antibacterial Potential of Caesalpinia coriaria (Jacq) Willd Fruit against Aeromonas spp. of Aquaculture Importance. Animals 2022, 12, 511. https://doi.org/10.3390/ani12040511
Rangel-López L, Rivero-Perez N, Valladares-Carranza B, Olmedo-Juárez A, Delgadillo-Ruiz L, Vega-Sánchez V, Hori-Oshima S, Nassan MA, Batiha GE-S, Zaragoza-Bastida A. Antibacterial Potential of Caesalpinia coriaria (Jacq) Willd Fruit against Aeromonas spp. of Aquaculture Importance. Animals. 2022; 12(4):511. https://doi.org/10.3390/ani12040511
Chicago/Turabian StyleRangel-López, Lenin, Nallely Rivero-Perez, Benjamín Valladares-Carranza, Agustín Olmedo-Juárez, Lucía Delgadillo-Ruiz, Vicente Vega-Sánchez, Sawako Hori-Oshima, Mohamed A. Nassan, Gaber El-Saber Batiha, and Adrian Zaragoza-Bastida. 2022. "Antibacterial Potential of Caesalpinia coriaria (Jacq) Willd Fruit against Aeromonas spp. of Aquaculture Importance" Animals 12, no. 4: 511. https://doi.org/10.3390/ani12040511