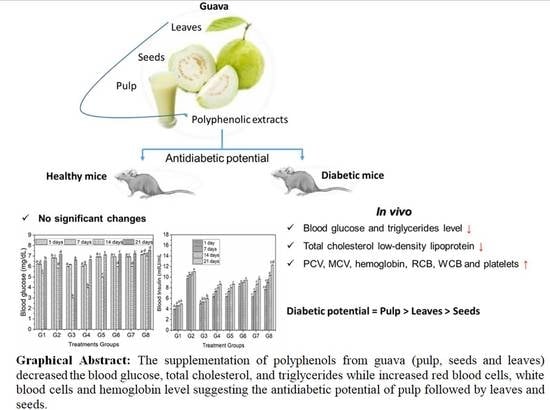
In Vivo Screening and Antidiabetic Potential of Polyphenol Extracts from Guava Pulp, Seeds and Leaves
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Samples
2.2. Proximate Composition
2.3. Extraction of Polyphenols
2.4. Total Polyphenol and DPPH Radical Scavenging Activity Assay
2.5. Experimental Design for Biological Studies
2.6. Physiological Parameters
2.7. Blood Serum and Biochemical Analysis
2.8. Statistical Analysis
3. Results
3.1. Proximate Analysis
3.2. Polyphenols of Guava Parts
3.3. Feed intake and Body Weight
3.4. Serum Lipid Profile
3.5. Hematological Studies
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- IFD. IDF Diabetes Atlas, 9th ed.; International Diabetes Federation: Brussels, Belgium, 2019. [Google Scholar]
- Finley, J.; Jaacks, L.; Peters, C.J.; Ort, D.R.; Aimone, A.M.; Conrad, Z.; Raiten, D.J. Perspective: Understanding the Intersection of Climate/Environmental Change, Health, Agriculture, and Improved Nutrition—A Case Study: Type 2 Diabetes. Adv. Nutr. 2019, 10, 731–738. [Google Scholar] [CrossRef]
- Dujic, T.; Causevic, A.; Bego, T.; Malenica, M.; Velija-Asimi, Z.; Pearson, E.R.; Semiz, S. Organic cation transporter 1 variants and gastrointestinal side effects of metformin in patients with Type 2 diabetes. Diabet. Med. 2015, 33, 511–514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kenneth, E.; Paul, T.; Istifanus, N.; Uba, U.; Rejoice, A.; Victor, O.; Mohammed, S. Phytochemical analysis and antibacterial activity of Psidium guajava L. leaf extracts. GSC Biol. Pharm. Sci. 2017, 1, 3–19. [Google Scholar]
- Lim, Y.Y.; Lim, T.T.; Tee, J.J. Antioxidant properties of guava fruit: Comparison with some local fruits. Sunway Acad. J. 2006, 3, 9–20. [Google Scholar]
- Zhu, X.; Ouyang, W.; Lan, Y.; Xiao, H.; Tang, L.; Liu, G.; Feng, K.; Zhang, L.; Song, M.; Cao, Y. Anti-hyperglycemic and liver protective effects of flavonoids from Psidium guajava L. (guava) leaf in diabetic mice. Food Biosci. 2020, 35, 100574. [Google Scholar] [CrossRef]
- Eidenberger, T.; Selg, M.; Krennhuber, K. Inhibition of dipeptidyl peptidase activity by flavonol glycosides of guava (Psidium guajava L.): A key to the beneficial effects of guava in type II diabetes mellitus. Fitoter. 2013, 89, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Abbas, M.; Saeed, F.; Anjum, F.M.; Afzaal, M.; Tufail, T.; Bashir, M.S.; Ishtiaq, A.; Hussain, S.; Suleria, H.A.R. Natural polyphenols: An overview. Int. J. Food Prop. 2016, 20, 1689–1699. [Google Scholar] [CrossRef] [Green Version]
- Jiménez-Escrig, A.; Rincón, M.; Pulido, R.; Saura-Calixto, F. Guava fruit (Psidium guajava L.) as a new source of antioxidant dietary fiber. J. Agric. Food Chem. 2001, 49, 5489–5493. [Google Scholar] [CrossRef]
- Joseph, B.; Priya, M. Review on nutritional, medicinal and pharmacological properties of guava (Psidium guajava Linn.). Int. J. Pharma Bio Sci. 2011, 2, 53–69. [Google Scholar]
- Yang, X.; Kong, F. Effects of tea polyphenols and different teas on pancreatic α-amylase activity in vitro. LWT 2016, 66, 232–238. [Google Scholar] [CrossRef]
- Xiao, J.; Ni, X.; Kai, G.; Chen, X. A Review on Structure–Activity Relationship of Dietary Polyphenols Inhibiting α-Amylase. Crit. Rev. Food Sci. Nutr. 2013, 53, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Horwitz, W.; Chichilo, P.; Reynolds, H.; AOAC. Official Methods of Analysis of the Association of Official Analytical Chemists; AOAC: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Both, S.; Chemat, F.; Strube, J. Extraction of polyphenols from black tea—Conventional and ultrasound assisted extraction. Ultrason. Sonochem. 2014, 21, 1030–1034. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, M.N.; Stecher, G.; Bonn, G.K. Quantification of polyphenolic compounds and flavonoids in Achillea millefolium and Equisetum arvense. Pak. J. Pharm. Sci. 2016, 29, 1519–1523. [Google Scholar] [PubMed]
- Safdar, M.N.; Kausar, T.; Jabbar, S.; Mumtaz, A.; Ahad, K.; Saddozai, A.A. Extraction and quantification of polyphenols from kinnow (Citrus reticulate L.) peel using ultrasound and maceration techniques. J. Food Drug Anal. 2017, 25, 488–500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, X.; Zhou, X.; Sun, W.; Zhang, L.; Zhang, C.; Zhang, X. Anti-diabetic effect of the polyphenol-rich extract from Tadehagi triquetrum in diabetic mice. Trop. J. Pharm. Res. 2020, 19, 829–835. [Google Scholar] [CrossRef]
- Johnston, K.L.; Clifford, M.N.; Morgan, L.M. Possible role for apple juice phenolic compounds in the acute modification of glucose tolerance and gastrointestinal hormone secretion in humans. J. Sci. Food Agric. 2002, 82, 1800–1805. [Google Scholar] [CrossRef]
- Sul’Ain, M.D.; Zazali, K.E.; Ahmad, N. Screening on Anti-Proliferative Activity of Psidium Guajava Leaves Extract towards Selected Cancer Cell Lines. J. US-China Med Sci. 2012, 9, 30–37. [Google Scholar] [CrossRef]
- Laily, N.; Kusumaningtyas, R.W.; Sukarti, I.; Rini, M.R.D.K. The Potency of Guava Psidium Guajava (L.) Leaves as a Functional Immunostimulatory Ingredient. Procedia Chem. 2015, 14, 301–307. [Google Scholar] [CrossRef] [Green Version]
- Lin, D.; Xiao, M.; Zhao, J.; Li, Z.; Xing, B.; Li, X.; Kong, M.; Li, L.; Zhang, Q.; Liu, Y.; et al. An Overview of Plant Phenolic Compounds and Their Importance in Human Nutrition and Management of Type 2 Diabetes. Molecules 2016, 21, 1374. [Google Scholar] [CrossRef]
- Boonpangrak, S.; Lalitmanat, S.; Suwanwong, Y.; Prachayasittikul, S.; Prachayasittikul, V. Analysis of Ascorbic Acid and Isoascorbic Acid in Orange and Guava Fruit Juices Distributed in Thailand by LC-IT-MS/MS. Food Anal. Methods 2015, 9, 1616–1626. [Google Scholar] [CrossRef]
- Linsel-Nitschke, P.; Tall, A.R. HDL as a target in the treatment of atherosclerotic cardiovascular disease. Nat. Rev. Drug Discov. 2005, 4, 193–205. [Google Scholar] [CrossRef] [PubMed]
- Arca, M.; Pigna, G.; Favoccia, C. Mechanisms of diabetic dyslipidemia: Relevance for atherogenesis. Curr. Vasc. Pharmacol. 2012, 10, 684–686. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Sun, J.; Yi, Q.; Wang, X.; Ju, X. Protective Effect of Polyphenols Extract of Adlay (Coix lachryma-jobi L. var. ma-yuen Stapf) on Hypercholesterolemia-Induced Oxidative Stress in Rats. Molecules 2012, 17, 8886–8897. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chambers, K.F.; Day, P.E.; Aboufarrag, H.T.; Kroon, P. Polyphenol Effects on Cholesterol Metabolism via Bile Acid Biosynthesis, CYP7A1: A Review. Nutrients 2019, 11, 2588. [Google Scholar] [CrossRef] [Green Version]
- Evans, R.A.; Frese, M.; Romero, J.; Cunningham, J.H.; Mills, K. Chronic fructose substitution for glucose or sucrose in food or beverages has little effect on fasting blood glucose, insulin, or triglycerides: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2017, 106, 519–529. [Google Scholar] [CrossRef]
- Nyambe-Silavwe, H.; Williamson, G. Polyphenol- and fibre-rich dried fruits with green tea attenuate starch-derived postprandial blood glucose and insulin: A randomised, controlled, single-blind, cross-over intervention. Br. J. Nutr. 2016, 116, 443–450. [Google Scholar] [CrossRef]
- Seifert, R. Drugs for Treatment of Diabetes Mellitus, in Basic Knowledge of Pharmacology; Springer: Berlin/Heidelberg, Germany, 2019; pp. 241–251. [Google Scholar]
- Schulze, C.; Bangert, A.; Kottra, G.; Geillinger, K.E.; Schwanck, B.; Vollert, H.; Blaschek, W.; Daniel, H. Inhibition of the intestinal sodium-coupled glucose transporter 1 (SGLT1) by extracts and polyphenols from apple reduces postprandial blood glucose levels in mice and humans. Mol. Nutr. Food Res. 2014, 58, 1795–1808. [Google Scholar] [CrossRef]
- Linderborg, K.M.; Järvinen, R.; Lehtonen, H.-M.; Viitanen, M.; Kallio, H. The fiber and/or polyphenols present in lingonberries null the glycemic effect of the sugars present in the berries when consumed together with added glucose in healthy human volunteers. Nutr. Res. 2012, 32, 471–478. [Google Scholar] [CrossRef]
- Castro-Acosta, M.L.; Stone, S.G.; Mok, J.E.; Mhajan, R.K.; Fu, C.-I.; Lenihan-Geels, G.N.; Corpe, C.P.; Hall, W.L. Apple and blackcurrant polyphenol-rich drinks decrease postprandial glucose, insulin and incretin response to a high-carbohydrate meal in healthy men and women. J. Nutr. Biochem. 2017, 49, 53–62. [Google Scholar] [CrossRef] [Green Version]
- Huyut, Z.; Şekeroğlu, M.R.; Balahoroğlu, R.; Huyut, M.T. Characteristics of resveratrol and serotonin on antioxidant capacity and susceptibility to oxidation of red blood cells in stored human blood in a time-dependent manner. J. Int. Med Res. 2017, 46, 272–283. [Google Scholar] [CrossRef]
- Mishra, N. Haematological and hypoglycemic potential Anethum graveolens seeds extract in normal and diabetic Swiss albino mice. Veter-World 2013, 6, 502. [Google Scholar] [CrossRef]
- Magrone, T.; Jirillo, E.; Spagnoletta, A.; Magrone, M.; Russo, M.A.; Fontana, S.; Laforgia, F.; Donvito, I.; Campanella, A.; Silvestris, F. Immune profile of obese people and in vitro effects of red grape polyphenols on peripheral blood mononuclear cells. Oxid. Med. Cell. Longev. 2017, 2017. [Google Scholar] [CrossRef] [Green Version]
- Varricchio, E.; Coccia, E.; Orso, G.; Lombardi, V.; Imperatore, R.; Vito, P.; Paolucci, M. Influence of polyphenols from olive mill wastewater on the gastrointestinal tract, alveolar macrophages and blood leukocytes of pigs. Ital. J. Anim. Sci. 2019, 18, 574–586. [Google Scholar] [CrossRef] [Green Version]



| Samples/Parameters | Leaves | Seeds | Pulp |
|---|---|---|---|
| Moisture (%) | 82.47 ± 2.10 b | 46.22 ± 1.50 a | 52.18± 1.60 ab |
| Ash (%) | 3.64 ± 0.05 c | 3.15 ± 0.10 b | 2.42 ± 0.20 a |
| Fat (%) | 0.62 ± 0.23 a | 7.94 ± 1.23 b | 6.54 ± 1.11 b |
| Protein (%) | 18.53 ± 2.29 b | 13.31 ± 1.34 a | 10.64 ± 1.66 a |
| Carbohydrates (%) | 12.74 ± 1.87 c | 3.06 ± 1.47 a | 8.57 ± 1.52 b |
| Vit. C (mg) | 103.05 ± 4.59 b | 87.43 ± 5.72 a | 116.17 ± 6.32 c |
| Total phenolic compounds (mgGAE/g) | 1717 ± 6.43 b | 344 ± 3.77 a | 383 ± 9.32 a |
| Antioxidant activity (%) | 234 ± 7.57 b | 89 ± 6.11 a | 365 ± 8.65 c |
| Groups | Total Cholesterol (mg/dL) | High Density Lipoprotein (HDL) (mg/dL) | Low Density Lipoproteins (LDL) (mg/dL) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 7 | 14 | 21 | 0 | 7 | 14 | 21 | 0 | 7 | 14 | 21 | |
| G1 | 57.1 a | 57.8 a | 57.8 a | 57.9 a | 22.6 a | 22.6 a | 22.3 b | 22.6 b | 23.4 a | 23.5 d | 23.4 d | 23.4 d |
| G2 | 57.8 b | 57.8 a | 56.9 b | 54.3 b | 22.0 b | 20.6 a | 19.6 a | 19.3 a | 23.5 a | 23.5 d | 23.4 d | 23.6 d |
| G3 | 57.6 b | 54.7 b | 54.2 b | 53.8 c | 22.0 b | 24.3 b | 26.0 c | 28.3 d | 22.5 a | 22.6 c | 21.9 c | 21.7 b |
| G4 | 57.6 b | 54.0 b | 54.8 b | 52.3 d | 22.0 b | 25.3 b | 26.3 c | 30.3 e | 22.5 a | 22.6 c | 21.9 c | 17.0 a |
| G5 | 57.2 b | 54.6 b | 53.4 b | 53.8 c | 22.0 b | 25.6 b | 27.0 d | 27.3 d | 23.4 a | 19.6 b | 19.2 b | 21.6 b |
| G6 | 57.4 b | 53.9 c | 53.9 c | 53.7 c | 22.3 b | 25.6 c | 27.6 d | 30.3 e | 23.2 a | 19.3 a | 16.8 a | 22.6 c |
| G7 | 57.6 b | 54.7 b | 54.9 b | 53.8 c | 22.0b | 24.6 b | 25.3 c | 25.3 c | 23.4 a | 22.0 c | 21.8 c | 21.1 b |
| G8 | 57.6 b | 53.4 c | 52.8 c | 54.9 b | 22.3 b | 25.3 c | 26.3 c | 28.3 d | 23.2 a | 21.8 c | 21.1 c | 21.0 b |
| Groups | PCV (%) | MCV (g/kg) | Hemoglobin Level (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 7 | 14 | 21 | 0 | 7 | 14 | 21 | 0 | 7 | 14 | 21 | |
| G1 | 38.6 a | 39.3 a | 40.3 a | 41.0 a | 92.6 a | 92.6 a | 94.3 a | 94.6 a | 10.0 b | 10.6 b | 11.3 b | 12.0 b |
| G2 | 39.3 b | 39.6 a | 40.6 a | 41.6 b | 94.0 b | 92.6 b | 94.3 a | 98.0 b | 9.0 a | 10.0 a | 10.0 a | 10.6 a |
| G3 | 39.3 b | 40.3 b | 41.0 b | 44.6c | 94.0 b | 97.0 c | 98.0 c | 99.0 c | 9.6 a | 11.6 b | 11.6 c | 12.3 c |
| G4 | 39.3 b | 40.6 b | 41.3 b | 46.0d | 94.0 b | 97.6 c | 99.3 cd | 106.0 f | 9.3 a | 11.8 c | 11.9 e | 12.9 f |
| G5 | 39.4 b | 42.6 d | 44.3 c | 42.0a | 94.0 b | 97.6 c | 103.3 d | 108.6 d | 9.3 a | 11.8 c | 11.8 d | 12.6 d |
| G6 | 39.3 b | 41.6 c | 44.3 c | 45.0c | 94.6 b | 102.3 e | 106.6 e | 109.0 g | 9.6 a | 11.9 d | 11.9 e | 12.7 de |
| G7 | 39.2 b | 40.3 b | 41.0 b | 41.6 b | 94.6 b | 97.6 c | 99.3 cd | 101.0 d | 9.6 a | 11.6 b | 11.9 e | 12.3 c |
| G8 | 39.5 b | 40.6 b | 41.3 b | 42.0 a | 94.3 b | 98.0 d | 100.6 d | 102.6 e | 9.3 a | 11.1 cd | 11.9 e | 12.8 e |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shabbir, H.; Kausar, T.; Noreen, S.; Rehman, H.u.; Hussain, A.; Huang, Q.; Gani, A.; Su, S.; Nawaz, A. In Vivo Screening and Antidiabetic Potential of Polyphenol Extracts from Guava Pulp, Seeds and Leaves. Animals 2020, 10, 1714. https://doi.org/10.3390/ani10091714
Shabbir H, Kausar T, Noreen S, Rehman Hu, Hussain A, Huang Q, Gani A, Su S, Nawaz A. In Vivo Screening and Antidiabetic Potential of Polyphenol Extracts from Guava Pulp, Seeds and Leaves. Animals. 2020; 10(9):1714. https://doi.org/10.3390/ani10091714
Chicago/Turabian StyleShabbir, Hassan, Tusneem Kausar, Sobia Noreen, Hafeez ur Rehman, Ashiq Hussain, Qingrong Huang, Adil Gani, Shiwei Su, and Asad Nawaz. 2020. "In Vivo Screening and Antidiabetic Potential of Polyphenol Extracts from Guava Pulp, Seeds and Leaves" Animals 10, no. 9: 1714. https://doi.org/10.3390/ani10091714