Green Tea and Pomegranate Extract Administered During Critical Moments of the Production Cycle Improves Blood Antiradical Activity and Alters Cecal Microbial Ecology of Broiler Chickens
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
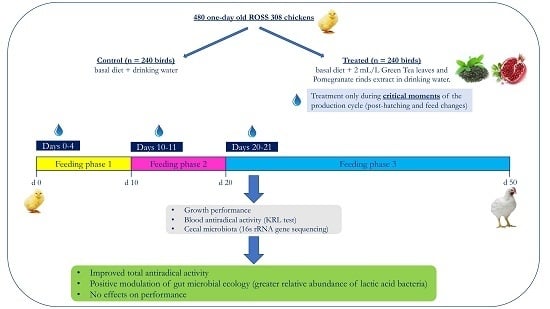
2.1. Animals and Housing
2.2. Experimental Design
2.3. Growth Performance and Water Intake
2.4. Total Antiradical Activity
2.5. Cecal Microbiota
2.6. Statistical Analysis
3. Results
3.1. Growth Performance, Water Intake, Carcass Characteristics and Total Antiradical Activity
3.2. Cecal Microbiota
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- European Union. Regulation (EC) No. 1831/2003 of the European Parliament and of the Council of 22 September 2003 on additives for use in animal nutrition. Off. J. Eur. Union 2003, 268, 29–43. [Google Scholar]
- Granstad, S.; Kristoffersen, A.B.; Benestad, S.L.; Sjurseth, S.K.; David, B.; Sorensen, L.; Fjermedal, A.; Edvardsen, D.H.; Sanson, G.; Lovland, A.; et al. Effect of Feed Additives as Alternatives to In-feed Antimicrobials on Production Performance and Intestinal Clostridium perfringens Counts in Broiler Chickens. Animals 2020, 10, 240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maria Cardinal, K.; Kipper, M.; Andretta, I.; Machado Leal Ribeiro, A. Withdrawal of antibiotic growth promoters from broiler diets: Performance indexes and economic impact. Poult. Sci. 2019, 98, 6659–6667. [Google Scholar] [CrossRef] [PubMed]
- Allen, H.K.; Levine, U.Y.; Looft, T.; Bandrick, M.; Casey, T.A. Treatment, promotion, commotion: Antibiotic alternatives in food-producing animals. Trends Microbiol. 2013, 21, 114–119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, G.; Hao, H.; Xie, S.; Wang, X.; Dai, M.; Huang, L.; Yuan, Z. Antibiotic alternatives: The substitution of antibiotics in animal husbandry? Front. Microbiol. 2014, 5, 217. [Google Scholar] [CrossRef] [Green Version]
- Markowiak, P.; Slizewska, K. The role of probiotics, prebiotics and synbiotics in animal nutrition. Gut Pathog. 2018, 10, 21. [Google Scholar] [CrossRef] [PubMed]
- Agazzi, A.; Tirloni, E.; Stella, S.; Maroccolo, S.; Ripamonti, B.; Bersani, C.; Caputo, J.M.; Dell’Orto, V.; Rota, N.; Savoini, G. Effects of Species-Specific Probiotic Addition to Milk Replacer on Calf Health and Performance during the First Month of Life. Ann. Anim. Sci. 2014, 14, 101–115. [Google Scholar] [CrossRef] [Green Version]
- Angwech, H.; Tavaniello, S.; Ongwech, A.; Kaaya, A.N.; Maiorano, G. Efficacy of In Ovo Delivered Prebiotics on Growth Performance, Meat Quality and Gut Health of Kuroiler Chickens in the Face of a Natural Coccidiosis Challenge. Animals 2019, 9, 876. [Google Scholar] [CrossRef] [Green Version]
- Anadón, A.; Ares, I.; Martínez-Larrañaga, M.R.; Martínez, M.A. Prebiotics and probiotics in feed and animal health. In Nutraceuticals in Veterinary Medicine; Springer Nature Switzerland AG: Cham, Switzerland, 2019; pp. 261–285. [Google Scholar]
- Tugnoli, B.; Giovagnoni, G.; Piva, A.; Grilli, E. From Acidifiers to Intestinal Health Enhancers: How Organic Acids Can Improve Growth Efficiency of Pigs. Animals 2020, 10, 134. [Google Scholar] [CrossRef] [Green Version]
- Khan, S.H.; Iqbal, J. Recent advances in the role of organic acids in poultry nutrition. J. Appl. Anim. Res. 2016, 44, 359–369. [Google Scholar] [CrossRef]
- Murugesan, G.R.; Syed, B.; Haldar, S.; Pender, C. Phytogenic Feed Additives as an Alternative to Antibiotic Growth Promoters in Broiler Chickens. Front. Vet. Sci. 2015, 2, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Windisch, W.; Schedle, K.; Plitzner, C.; Kroismayr, A. Use of phytogenic products as feed additives for swine and poultry. J. Anim. Sci. 2008, 86, E140–E148. [Google Scholar] [CrossRef] [PubMed]
- Lillehoj, H.; Liu, Y.; Calsamiglia, S.; Fernandez-Miyakawa, M.E.; Chi, F.; Cravens, R.L.; Oh, S.; Gay, C.G. Phytochemicals as antibiotic alternatives to promote growth and enhance host health. Vet. Res. 2018, 49, 76. [Google Scholar] [CrossRef] [Green Version]
- Brenes, A.; Roura, E. Essential oils in poultry nutrition: Main effects and modes of action. Anim. Feed Sci. Technol. 2010, 158, 1–14. [Google Scholar] [CrossRef]
- Ognik, K.; Cholewinska, E.; Sembratowicz, I.; Grela, E.; Czech, A. The potential of using plant antioxidants to stimulate antioxidant mechanisms in poultry. World Poult. Sci. J. 2016, 72, 291–298. [Google Scholar] [CrossRef]
- Duenas, M.; Munoz-Gonzalez, I.; Cueva, C.; Jimenez-Giron, A.; Sanchez-Patan, F.; Santos-Buelga, C.; Moreno-Arribas, M.V.; Bartolome, B. A survey of modulation of gut microbiota by dietary polyphenols. Biomed. Res. Int. 2015, 2015, 850902. [Google Scholar] [CrossRef]
- Kers, J.G.; Velkers, F.C.; Fischer, E.A.J.; Hermes, G.D.A.; Stegeman, J.A.; Smidt, H. Host and Environmental Factors Affecting the Intestinal Microbiota in Chickens. Front. Microbiol. 2018, 9, 235. [Google Scholar] [CrossRef] [Green Version]
- Ballou, A.L.; Ali, R.A.; Mendoza, M.A.; Ellis, J.C.; Hassan, H.M.; Croom, W.J.; Koci, M.D. Development of the Chick Microbiome: How Early Exposure Influences Future Microbial Diversity. Front. Vet. Sci. 2016, 3, 2. [Google Scholar] [CrossRef] [Green Version]
- Hamady, G.A.; Abdel-Moneim, M.A.; El-Chaghaby, G.A.; Abd-El-Ghany, Z.M.; Hassanin, M.S. Effect of Pomegranate peel extract as natural growth promoter on the productive performance and intestinal microbiota of broiler chickens. Afr. J. Agric. Sci. Technol. 2015, 3, 514–519. [Google Scholar]
- Jelveh, K.; Rasouli, B.; Seidavi, A.; Diarra, S.S. Comparative effects of Chinese green tea (Camellia sinensis) extract and powder as feed supplements for broiler chickens. J. Appl. Anim. Res. 2018, 46, 1114–1117. [Google Scholar] [CrossRef] [Green Version]
- Saleh, H.; Golian, A.; Kermanshahi, H.; Mirakzehi, M.T. Antioxidant status and thigh meat quality of broiler chickens fed diet supplemented with alpha-tocopherolacetate, pomegranate pomace and pomegranate pomace extract. Ital. J. Anim. Sci. 2018, 17, 386–395. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Ni, J.; Li, H. Effect of green tea and mulberry leaf powders on the gut microbiota of chicken. BMC Vet. Res. 2019, 15, 77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chacko, S.M.; Thambi, P.T.; Kuttan, R.; Nishigaki, I. Beneficial effects of green tea: A literature review. Chin. Med. 2010, 5, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, S.H. The use of green tea (Camellia sinensis) as a phytogenic substance in poultry diets. Onderstepoort J. Vet. 2014, 81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, B.N.; Shankar, S.; Srivastava, R.K. Green tea catechin, epigallocatechin-3-gallate (EGCG): Mechanisms, perspectives and clinical applications. Biochem. Pharmacol. 2011, 82, 1807–1821. [Google Scholar] [CrossRef] [Green Version]
- Zarfeshany, A.; Asgary, S.; Javanmard, S.H. Potent health effects of pomegranate. Adv. Biomed. Res. 2014, 3, 100. [Google Scholar] [CrossRef]
- Jain, D.P.; Pancholi, S.S.; Patel, R. Synergistic antioxidant activity of green tea with some herbs. J. Adv. Pharm. Technol. Res. 2011, 2, 177–183. [Google Scholar] [CrossRef]
- Saeed, M.; Xu, Y.T.; Zhang, T.T.; Qian, R.; Chao, S. 16S ribosomal RNA sequencing reveals a modulation of intestinal microbiome and immune response by dietary L-theanine supplementation in broiler chickens. Poult. Sci. 2019, 98, 842–854. [Google Scholar] [CrossRef]
- Zou, X.; Xiao, R.; Li, H.; Liu, T.; Liao, Y.; Wang, Y.; Wu, S.; Li, Z. Effect of a novel strain of Lactobacillus brevis M8 and tea polyphenol diets on performance, meat quality and intestinal microbiota in broilers. Ital. J. Anim. Sci. 2018, 17, 396–407. [Google Scholar] [CrossRef] [Green Version]
- Rowghani, E.; Tabeidian, S.; Abolfathi, E. The effects of green tea extract and vitamin E on the growth performance and immune response in broiler chicks. Res. Opin. Anim. Vet. Sci. 2016, 6, 200–205. [Google Scholar]
- Kaneko, K.; Yamasaki, K.; Tagawa, Y.; Tokunaga, M.; Tobisa, M.; Furuse, M. Effects of Japanese tea (green tea) on the growth and fat deposition of the broiler. Jpn. Poult. Sci. 2000, 37, 349–356. [Google Scholar] [CrossRef] [Green Version]
- AOAC. Official Methods of Analysis; Association Official Analytical Chemists: Arlington, VA, USA, 2000. [Google Scholar]
- Caspar-Bauguil, S.; Maestre, N.; Segafredo, C.; Galinier, A.; Garcia, J.; Prost, M.; Périquet, B.; Pénicaud, L.; Salvayre, R.; Casteilla, L. Evaluation of whole antioxidant defenses of human mononuclear cells by a new in vitro biological test: Lack of correlation between erythrocyte and mononuclear cell resistance to oxidative stress. Clin. Biochem. 2009, 42, 510–514. [Google Scholar] [CrossRef] [PubMed]
- Lesgards, J.F.; Lehucher-Michel, M.P.; Vidal, N.; Prost, M.; Stocker, P. Assessment of antioxidative activity of lipid- and water-soluble vitamins in human whole blood. Comparative analysis between a biological test and chemical methods. Int. J. Vitam. Nutr. Res. 2005, 75, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Schokker, D.; Jansman, A.J.; Veninga, G.; de Bruin, N.; Vastenhouw, S.A.; de Bree, F.M.; Bossers, A.; Rebel, J.M.; Smits, M.A. Perturbation of microbiota in one-day old broiler chickens with antibiotic for 24 hours negatively affects intestinal immune development. BMC Genom. 2017, 18, 241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takahashi, S.; Tomita, J.; Nishioka, K.; Hisada, T.; Nishijima, M. Development of a prokaryotic universal primer for simultaneous analysis of Bacteria and Archaea using next-generation sequencing. PLoS ONE 2014, 9, e105592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glockner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res 2013, 41, D590–D596. [Google Scholar] [CrossRef]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef] [Green Version]
- Dhariwal, A.; Chong, J.; Habib, S.; King, I.L.; Agellon, L.B.; Xia, J. MicrobiomeAnalyst: A web-based tool for comprehensive statistical, visual and meta-analysis of microbiome data. Nucleic Acids Res 2017, 45, W180–W188. [Google Scholar] [CrossRef]
- Paulson, J.N.; Stine, O.C.; Bravo, H.C.; Pop, M. Differential abundance analysis for microbial marker-gene surveys. Nat. Methods 2013, 10, 1200–1202. [Google Scholar] [CrossRef] [Green Version]
- Ri, C.S.; Jiang, X.R.; Kim, M.H.; Wang, J.; Zhang, H.J.; Wu, S.G.; Bontempo, V.; Qi, G.H. Effects of dietary oregano powder supplementation on the growth performance, antioxidant status and meat quality of broiler chicks. Ital. J. Anim. Sci. 2017, 16, 246–252. [Google Scholar] [CrossRef] [Green Version]
- Ahasan, A.; Invernizzi, G.; Farina, G.; Pilotto, A.; Barbe, F.; Bontempo, V.; Rossi, R.; Bellagamba, F.; Lecchi, C.; Savoini, G.; et al. The effects of superoxide dismutase-rich melon pulp concentrate on inflammation, antioxidant status and growth performance of challenged post-weaning piglets. Animal 2019, 13, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Bontempo, V.; Jiang, X.R.; Cheli, F.; Lo Verso, L.; Mantovani, G.; Vitari, F.; Domeneghini, C.; Agazzi, A. Administration of a novel plant extract product via drinking water to post-weaning piglets: Effects on performance and gut health. Animal 2014, 8, 721–730. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils--a review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef] [PubMed]
- Farahat, M.; Abdallah, F.; Abdel-Hamid, T.; Hernandez-Santana, A. Effect of supplementing broiler chicken diets with green tea extract on the growth performance, lipid profile, antioxidant status and immune response. Br. Poult. Sci. 2016, 57, 714–722. [Google Scholar] [CrossRef]
- Erener, G.; Ocak, N.; Altop, A.; Cankaya, S.; Aksoy, H.M.; Ozturk, E. Growth performance, meat quality and caecal coliform bacteria count of broiler chicks fed diet with green tea extract. Asian Australas. J. Anim. Sci. 2011, 24, 1128–1135. [Google Scholar] [CrossRef]
- Ginsburg, I.; Kohen, R.; Koren, E. Microbial and host cells acquire enhanced oxidant-scavenging abilities by binding polyphenols. Arch. Biochem. Biophys. 2011, 506, 12–23. [Google Scholar] [CrossRef]
- Rao, S.V.R.; Raju, M.V.L.N.; Prakash, B.; Rajkumar, U.; Reddy, E.P.K. Effect of supplementing moringa (Moringa oleifera) leaf meal and pomegranate (Punica granatum) peel meal on performance, carcass attributes, immune and antioxidant responses in broiler chickens. Anim. Prod. Sci. 2019, 59, 288–294. [Google Scholar] [CrossRef]
- Apajalahti, J.; Kettunen, A.; Graham, H. Characteristics of the gastrointestinal microbial communities, with special reference to the chicken. World Poult. Sci. J. 2004, 60, 223–232. [Google Scholar] [CrossRef]
- Yin, Y.S.; Lei, F.; Zhu, L.Y.; Li, S.J.; Wu, Z.W.; Zhang, R.F.; Gao, G.F.; Zhu, B.L.; Wang, X. Exposure of different bacterial inocula to newborn chicken affects gut microbiota development and ileum gene expression. ISME J. 2010, 4, 367–376. [Google Scholar] [CrossRef] [Green Version]
- Gabriel, I.; Lessire, M.; Mallet, S.; Guillot, J.F. Microflora of the digestive tract: Critical factors and consequences for poultry. World Poult. Sci. J. 2006, 62, 499–511. [Google Scholar] [CrossRef]
- Wei, S.; Morrison, M.; Yu, Z. Bacterial census of poultry intestinal microbiome. Poult. Sci. 2013, 92, 671–683. [Google Scholar] [CrossRef] [PubMed]
- Muir, W.I.; Bryden, W.L.; Husband, A.J. Immunity, vaccination and the avian intestinal tract. Dev. Comp. Immunol. 2000, 24, 325–342. [Google Scholar] [CrossRef]
- Cao, L.; Yang, X.J.; Li, Z.J.; Sun, F.F.; Wu, X.H.; Yao, J.H. Reduced lesions in chickens with Clostridium perfringens-induced necrotic enteritis by Lactobacillus fermentum 1.2029. Poult. Sci. 2012, 91, 3065–3071. [Google Scholar] [CrossRef] [PubMed]
- Messaoudi, S.; Kergourlay, G.; Dalgalarrondo, M.; Choiset, Y.; Ferchichi, M.; Prevost, H.; Pilet, M.F.; Chobert, J.M.; Manai, M.; Dousset, X. Purification and characterization of a new bacteriocin active against Campylobacter produced by Lactobacillus salivarius SMXD51. Food Microbiol. 2012, 32, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Duncan, S.H.; Hold, G.L.; Barcenilla, A.; Stewart, C.S.; Flint, H.J. Roseburia intestinalis sp. nov., a novel saccharolytic, butyrate-producing bacterium from human faeces. Int. J. Syst. Evol. Microbiol. 2002, 52, 1615–1620. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.C.; Kil, D.Y.; Sul, W.J. Cecal microbiome divergence of broiler chickens by sex and body weight. J. Microbiol. 2017, 55, 939–945. [Google Scholar] [CrossRef]

| Ingredients (g/kg feed) | Starter Diet | Grower Diet | Finisher Diet |
|---|---|---|---|
| 0–10 days | 11–20 days | 21–50 days | |
| Corn | 550.5 | 574.0 | 616.7 |
| Soybean meal (48% crude protein) | 373.0 | 341.0 | 292.0 |
| Soybean oil | 30.0 | 43.0 | 53.0 |
| Dicalcium phosphate | 25.0 | 25.0 | 21.0 |
| Calcium carbonate | 7.0 | 4.5 | 5.0 |
| Mineral + vitamin premix † | 5.0 | 5.0 | 5.0 |
| Sodium chloride (NaCl) | 4.0 | 4.0 | 4.0 |
| DL-Methionine (DL-Met) | 3.2 | 1.8 | 1.6 |
| L-Lysine-HCl (L-Lys-HCl) | 2.3 | 1.7 | 1.7 |
| Nutrient values of diets, analysed (g/kg) | |||
| Dry matter (g) | 877.7 | 878.2 | 878.0 |
| Crude protein (g) | 229.7 | 215.1 | 195.0 |
| Ether extract (g) | 56.3 | 69.4 | 79.8 |
| Ash (g) | 68.2 | 64.04 | 58.6 |
| Calcium (Ca; g) | 10.0 | 9.1 | 8.1 |
| Phosphorus (P; g) | 8.7 | 8.5 | 7.6 |
| Nutrient values of diets, calculated (g/kg) | |||
| Metabolizable energy (kcal/kg) | 3002.5 | 3099.9 | 3200.1 |
| Lysine (Lys) | 10.0 | 8.3 | 7.6 |
| Metionine + cysteine (Met + Cys) | 6.4 | 4.9 | 4.4 |
| Item | Groups | SEM | p-Value | |||
|---|---|---|---|---|---|---|
| C | T | Treatment | Time | Treatment × Time | ||
| No. Birds/Pen | 20 | 20 | ||||
| BW (kg/pen) 1 | ||||||
| 0 day | 0.883 | 0.872 | 0.842 | 0.469 | <0.001 | 0.638 |
| 10 day | 6.195 | 6.215 | ||||
| 20 day | 18.312 | 18.332 | ||||
| 50 day | 74.892 | 73.106 | ||||
| Gain (kg/pen) 1 | ||||||
| 0–10 days | 5.312 | 5.342 | 1.075 | 0.445 | <0.001 | 0.533 |
| 11–20 days | 12.117 | 12.117 | ||||
| 21–49 days | 56.580 | 54.774 | ||||
| TWG | 74.008 | 72.233 | 1.667 | 0.460 | ||
| FI (kg/pen) 1 | ||||||
| 0–10 days | 6.393 | 6.343 | 0.819 | 0.276 | <0.001 | 0.294 |
| 11–20 days | 18.102 | 18.158 | ||||
| 21–49 days | 122.808 | 120.863 | ||||
| TFI | 147.302 | 145.363 | 1.257 | 0.287 | ||
| FCR (kg/kg) 1 | ||||||
| 0–10 days | 1.20 | 1.19 | 0.035 | 0.721 | <0.001 | 0.689 |
| 11–20 days | 1.50 | 1.50 | ||||
| 21–49 days | 2.18 | 2.23 | ||||
| TFCR | 2.00 | 2.02 | 0.036 | 0.613 | ||
| Mortality (%) | 3.33 | 5.83 | 0.190 | |||
| Carcass characteristics | ||||||
| No. birds 2 | 12 | 12 | ||||
| Dressing (%) | 75.59 | 76.83 | 0.56 | 0.133 | ||
| Breast (%) | 21.41 | 22.41 | 0.66 | 0.293 | ||
| Groups | SEM | p-Value | ||
|---|---|---|---|---|
| Item | C | T | ||
| No. birds 1 | 12 | 12 | ||
| HT50 whole blood, min. | 69.17 | 76.52 | 4.91 | <0.001 |
| HT50 RBC, min. | 56.72 | 61.28 | 3.45 | 0.023 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perricone, V.; Comi, M.; Giromini, C.; Rebucci, R.; Agazzi, A.; Savoini, G.; Bontempo, V. Green Tea and Pomegranate Extract Administered During Critical Moments of the Production Cycle Improves Blood Antiradical Activity and Alters Cecal Microbial Ecology of Broiler Chickens. Animals 2020, 10, 785. https://doi.org/10.3390/ani10050785
Perricone V, Comi M, Giromini C, Rebucci R, Agazzi A, Savoini G, Bontempo V. Green Tea and Pomegranate Extract Administered During Critical Moments of the Production Cycle Improves Blood Antiradical Activity and Alters Cecal Microbial Ecology of Broiler Chickens. Animals. 2020; 10(5):785. https://doi.org/10.3390/ani10050785
Chicago/Turabian StylePerricone, Vera, Marcello Comi, Carlotta Giromini, Raffaella Rebucci, Alessandro Agazzi, Giovanni Savoini, and Valentino Bontempo. 2020. "Green Tea and Pomegranate Extract Administered During Critical Moments of the Production Cycle Improves Blood Antiradical Activity and Alters Cecal Microbial Ecology of Broiler Chickens" Animals 10, no. 5: 785. https://doi.org/10.3390/ani10050785