Maternal Exposure to T-2 Toxin Affects Puberty Genes and Delays Estrus Cycle in Mice Offspring
Abstract
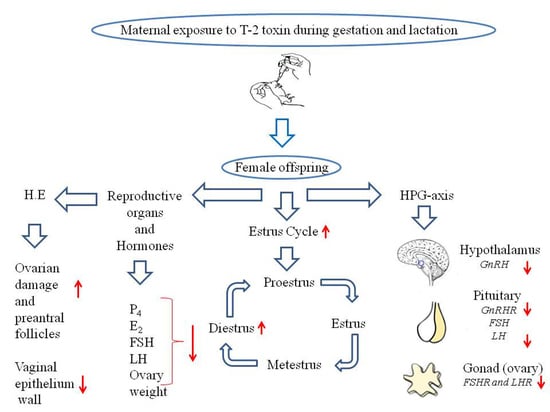
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Treatment
2.3. Experiment Design
2.4. Observation of Vaginal Opening
2.5. Vaginal Smear and Onset of Estrus Cycle Detection Procedure
2.6. Organ/Tissue Sampling
2.7. Hormone Assay
2.8. Haematoxylin and Eosin Staining
2.9. Oxidative Stress Markers
2.10. Examination of Female Reproductive Genes
2.11. Statistics
3. Results
3.1. T-2 Toxin Effects Through Maternal Exposure on Body Weight, Age at Different Intervals, and Length of Estrus Cycle of Mice Offspring
3.2. T-2 Toxin Effects Through Maternal Exposure on Different Stages of the Estrus Cycle of Mice Offspring
3.3. T-2 Toxin Effects Through Maternal Exposure on the Reproductive Organ Weight of Mice Offspring
3.4. T-2 Toxin Effects Through Maternal Exposure on Serum Hormone Level of Female Mice
3.5. T-2 Toxin Effects Through Maternal Exposure on Oxidative Stress Parameters of Mice Offspring
3.6. T-2 Toxin Effects Through Maternal Exposure on mRNA Expression in Hypothalamic, Pituitary, and Gonad (Ovarian) Axis
3.7. T-2 Toxin Effects Through Maternal Exposure on Histopathology of The Ovary and Vagina of Mice Offspring
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Afsah-Hejri, L.; Jinap, S.; Hajeb, P.; Radu, S.; Shakibazadeh, S. A review on mycotoxins in food and feed: Malaysia case study. Compr. Rev. Food Sci. Food Saf. 2013, 12, 629–651. [Google Scholar] [CrossRef]
- Bhat, R.; Rai, R.V.; Karim, A.A. Mycotoxins in food and feed: Present status and future concerns. Compr. Rev. Food Sci. Food Saf. 2010, 9, 57–81. [Google Scholar] [CrossRef]
- Wu, Q.; Dohnal, V.; Kuca, K.; Yuan, Z. Trichothecenes: Structure-toxic activity relationships. Curr. Drug Metab. 2013, 14, 641–660. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; van der Lee, T.; Verstappen, E.; van Gent, M.; Waalwijk, C. Targeting trichothecene biosynthetic genes. In Mycotoxigenic Fungi; Springer: Berlin/Heidelberg, Germany, 2017; pp. 173–189. [Google Scholar]
- Gruber-Dorninger, C.; Jenkins, T.; Schatzmayr, G. Global mycotoxin occurrence in feed: A ten-year survey. Toxins 2019, 11, 375. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Liu, S.; Zheng, H.; He, C.; Zhang, H. T-2 toxin, zearalenone and fumonisin b1 in feedstuffs from china. Food Addit. Contam. Part B 2013, 6, 116–122. [Google Scholar] [CrossRef]
- Milanez, T.V.; Valente-Soares, L.M.; Baptista, G.G. Occurrence of trichothecene mycotoxins in brazilian corn-based food products. Food Control 2006, 17, 293–298. [Google Scholar] [CrossRef]
- Omurtag, G.; Yazicioğlu, D. Occurrence of t-2 toxin in processed cereals and pulses in turkey determined by hplc and tlc. Food Addit. Contam. 2001, 18, 844–849. [Google Scholar] [CrossRef]
- Ishigami, N.; Sehata, S. T-2 toxin-induced toxicity in pregnant mice and rats. Int. J. Mol. Sci. 2008, 9, 2146–2158. [Google Scholar]
- Scientific Committee on Food. Part 6: Group evaluation of T-2 toxin, HT-2 toxin, nivalenol anddeoxynivalenol. In Opinion of the Scientific Committee on Food on Fusarium Toxins; European Commission: Brussel, Belgium, 2002; pp. 1–12. [Google Scholar]
- Yuan, Z.; Matias, F.B.; Yi, J.-E.; Wu, J. T-2 toxin-induced cytotoxicity and damage on tm3 leydig cells. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2016, 181, 47–54. [Google Scholar] [CrossRef]
- Jian Ying, Y.; Yong Fa, Z.; Ai Min, L.; Xiang Feng, K.; Yuan Xiao, L.; Kai Wang, M.; Ai Hua, J.; Shu Ying, F.; Xiao Lan, Q. Toxic effects of t-2 toxin on reproductive system in male mice. Toxicol. Ind. Health 2009, 26, 25–31. [Google Scholar] [CrossRef]
- Chulani, V.L.; Gordon, L.P. Adolescent growth and development. Prim. Care Clin. Off. Pract. 2014, 41, 465–487. [Google Scholar]
- Lafarge-Frayssinet, C.; Chakor, K.; Lafont, P.; Frayssinet, C. Transplacental transfer of t2-toxin: Pathological effect. J. Environ. Pathol. Toxicol. Oncol. Off. Organ Int. Soc. Environ. Toxicol. Cancer 1990, 10, 64–68. [Google Scholar]
- Food and Agriculture Organization of the United Nations. Safety Evaluation of Certain Mycotoxins in Food; Food and Agriculture Organization of the United Nations: Rome, Italy, 2001. [Google Scholar]
- Schuhmacher-Wolz, U.; Heine, K.; Schneider, K. Report on toxicity data on trichothecene mycotoxins ht-2 and t-2 toxins. EFSA Supporting Publ. 2010, 7, 65E. [Google Scholar] [CrossRef]
- Ueno, Y. Toxicological features of t-2 toxin and related trichothecenes. Toxicol. Sci. 1984, 4, 124–132. [Google Scholar] [CrossRef]
- Byers, S.L.; Wiles, M.V.; Dunn, S.L.; Taft, R.A. Mouse estrous cycle identification tool and images. PLoS ONE 2012, 7, e35538. [Google Scholar] [CrossRef] [Green Version]
- Ren, X.; Li, R.; Liu, J.; Huang, K.; Wu, S.; Li, Y.; Li, C. Effects of glyphosate on the ovarian function of pregnant mice, the secretion of hormones and the sex ratio of their fetuses. Environ. Pollut. 2018, 243, 833–841. [Google Scholar] [CrossRef]
- Dänicke, S.; Brezina, U. Kinetics and metabolism of the fusarium toxin deoxynivalenol in farm animals: Consequences for diagnosis of exposure and intoxication and carry over. Food Chem. Toxicol. 2013, 60, 58–75. [Google Scholar] [CrossRef]
- Kinouchi, R.; Matsuzaki, T.; Iwasa, T.; Gereltsetseg, G.; Nakazawa, H.; Kunimi, K.; Kuwahara, A.; Yasui, T.; Irahara, M. Prepubertal exposure to glucocorticoid delays puberty independent of the hypothalamic kiss1-gnrh system in female rats. Int. J. Dev. Neurosci. 2012, 30, 596–601. [Google Scholar] [CrossRef]
- Yang, R.; Wang, Y.-M.; Zhang, L.-S.; Zhang, L.; Zhao, Z.-M.; Zhao, J.; Peng, S.-Q. Delay of the onset of puberty in female rats by prepubertal exposure to t-2 toxin. Toxins 2015, 7, 4668–4683. [Google Scholar] [CrossRef] [Green Version]
- Blakley, B.R.; Hancock, D.S.; Rousseaux, C.G. Embryotoxic effects of prenatal t-2 toxin exposure in mice. Can. J. Vet. Res. 1987, 51, 399. [Google Scholar]
- Sehata, S.; Teranishi, M.; Atsumi, F.; Uetsuka, K.; Nakayama, H.; Doi, K. T-2 toxin-induced morphological changes in pregnant rats. J. Toxicol. Pathol. 2003, 16, 59–65. [Google Scholar] [CrossRef]
- Yuan, G.; Wang, Y.; Yuan, X.; Zhang, T.; Zhao, J.; Huang, L.; Peng, S. T-2 toxin induces developmental toxicity and apoptosis in zebrafish embryos. J. Environ. Sci. 2014, 26, 917–925. [Google Scholar] [CrossRef]
- Cortinovis, C.; Pizzo, F.; Spicer, L.J.; Caloni, F. Fusarium mycotoxins: Effects on reproductive function in domestic animals—A review. Theriogenology 2013, 80, 557–564. [Google Scholar] [CrossRef]
- Ewuola, E.; Egbunike, G. Effects of dietary fumonisin b1 on the onset of puberty, semen quality, fertility rates and testicular morphology in male rabbits. Reproduction 2010, 139, 439–445. [Google Scholar] [CrossRef] [Green Version]
- Sehata, S.; Kiyosawa, N.; Sakuma, K.; Ito, K.; Yamoto, T.; Teranishi, M.; Uetsuka, K.; Nakayama, H.; Doi, K. Gene expression profiles in pregnant rats treated with t-2 toxin. Exp. Toxicol. Pathol. 2004, 55, 357–366. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, L.; Wang, Y.; Wu, R.; Hua, C.; Zhao, J.; Liu, Y.; Peng, S. Effects of low dose t-2 toxin on secretion of gonadotropin-releasing hormone in the immortalized hypothalamic gt1-7 cell line. Toxicon 2015, 100, 67–72. [Google Scholar] [CrossRef]
- Li, X.; Sun, Z.; Manthari, R.K.; Li, M.; Guo, Q.; Wang, J. Effect of gestational exposure to arsenic on puberty in offspring female mice. Chemosphere 2018, 202, 119–126. [Google Scholar] [CrossRef]
- Gervásio, C.G.; Bernuci, M.P.; Silva-de-Sá, M.F.; Rosa-e-Silva, A.C.J.D.S. The role of androgen hormones in early follicular development. ISRN Obstet. Gynecol. 2014, 2014. [Google Scholar] [CrossRef] [Green Version]
- Maruniakova, N.; Kadasi, A.; Sirotkin, A.V.; Bulla, J.; Kolesarova, A. T-2 toxin and its metabolite ht-2 toxin combined with insulin-like growth factor-i modify progesterone secretion by porcine ovarian granulosa cells. J. Environ. Sci. Health Part A 2014, 49, 404–409. [Google Scholar] [CrossRef]





| Gene | Accession | Primers | Size | Product (bp) |
|---|---|---|---|---|
| Actb | NM_007393.5 | F: 5’-CTGACCGAGCGTGGCTACAG-3’ R: 5’-CAGTGGCCATCTCCTGCTCG-3’ | 20 | 112 |
| Gnrh | NM_008145.3 | F: 5′-CTGGTCCTATGGGTTGCGCC-3′ R: 5′-AACGGGGCCAGTGGACAGTA-3′ | 20 | 134 |
| Gnrhr | NM-014715.2 | F: 5′-GTATGCTGGAGAGTACTCTGCA-3′ R: 5′-GGATGATGAAGAGGCAGCTGAAG-3′ | 20 | 380 |
| Lhb | NM_008497.2 | F: 5′-TGCTGAGCCCAAGTGTGGTG-3′ R: 5′-AGGCACAGGAGGCAAAGCAG-3′ | 20 | 185 |
| Lhr | NM_013582.2 | F: 5′-TGCAGTGCAGCTGGCTTCTT-3′ R: 5′-CTGCTGACACCCACAAGGGG-3′ | 20 | 206 |
| Fshb | NM_008045.3 | F: 5′-GACCCAGCTCGGCCCAATAC-3′ R: 5′-CTCACGGTGCAGTCAGTGCT-3′ | 20 | 170 |
| Fshr | NM_013523.3 | F: 5′-TACAGCTCTGCCATGCTGCC-3′ R: 5′-GCGTTGAGTACGAGGAGGGC-3′ | 20 | 175 |
| Item | Control | T-2 toxin mg/kg Body Weight | |
|---|---|---|---|
| 0 | 0.005 | 0.05 | |
| Length of cycles | 4.57 ± 0.11 b | 4.80 ± 0.26 b | 5.79 ± 0.18 a |
| Proestrus | 1.00 ± 0.00 | 1.07 ± 0.07 | 1.13 ± 0.08 |
| Estrus | 1.00 ± 0.00 | 1.07 ± 0.04 | 0.96 ± 0.02 |
| Metestrus | 1.17 ± 0.07 | 1.23 ± 0.11 | 1.50 ± 0.13 |
| Diestrus | 1.40 ± 0.11 b | 1.43 ± 0.17 b | 2.20 ± 0.03 a |
| Reproductive Organs | Control | T-2 toxin mg/kg Body Weight | |
|---|---|---|---|
| 0 | 0.005 | 0.05 | |
| Ovary (mg) | 25.54 ± 1.56 a | 22.44 ± 1.01 a | 13.92 ± 1.22 b |
| Relative ovary (%) | 0.09 ± 0.01 a | 0.08 ± 0.01 a | 0.07 ± 0.01 b |
| Uterus (mg) | 114.00 ± 8.23 a | 111.70 ± 2.55 a | 35.42 ± 6.14 b |
| Relative uterus (%) | 0.38 ± 0.03 a | 0.41 ± 0.02 a | 0.17 ± 0.03 b |
| Uterus length (mm) | 11.45 ± 0.68 a | 12.13 ± 0.69 a | 14.18 ± 0.74 b |
| Uterus width (mm) | 1.78 ± 0.19 a | 1.64 ± 0.18 ab | 1.16 ± 0.09 b |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perveen, A.; Shen, J.; Ali Kaka, N.; Li, C. Maternal Exposure to T-2 Toxin Affects Puberty Genes and Delays Estrus Cycle in Mice Offspring. Animals 2020, 10, 471. https://doi.org/10.3390/ani10030471
Perveen A, Shen J, Ali Kaka N, Li C. Maternal Exposure to T-2 Toxin Affects Puberty Genes and Delays Estrus Cycle in Mice Offspring. Animals. 2020; 10(3):471. https://doi.org/10.3390/ani10030471
Chicago/Turabian StylePerveen, Aneela, Jiakun Shen, Niaz Ali Kaka, and Chunmei Li. 2020. "Maternal Exposure to T-2 Toxin Affects Puberty Genes and Delays Estrus Cycle in Mice Offspring" Animals 10, no. 3: 471. https://doi.org/10.3390/ani10030471