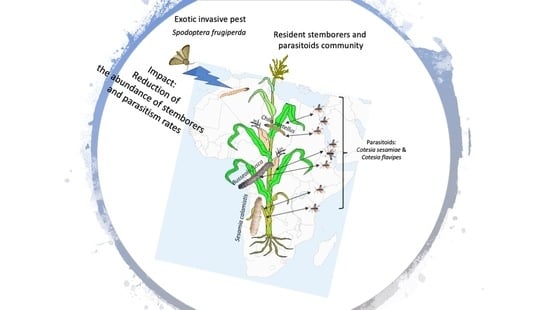
Impact of an Exotic Invasive Pest, Spodoptera frugiperda (Lepidoptera: Noctuidae), on Resident Communities of Pest and Natural Enemies in Maize Fields in Kenya
Abstract
:1. Introduction
2. Materials and Methods
2.1. Effect of FAW Introduction and Its Abundance on Stemborer Density and Parasitism across the Maize Agroecological Zones (AEZs) of Kenya
2.2. Effect of FAW Introduction and Its Abundance on Stemborer Density across Different Maize Phenological Stages
3. Results
3.1. Distribution and Abundance of the FAW in the Different AEZs of Kenya
3.2. Effect of FAW Introduction and Its Abundance on Stemborer Density and Parasitism across the Maize AEZs of Kenya
3.3. Effect of FAW Introduction and Its Abundance on Stemborer Density across Different Maize Phenological Stages
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A. Distribution and Abundance of the FAW and Stemborers in the Different AEZs of Kenya
| AEZs | Species | Before FAW | After FAW | Likelihood Ratio (LR) | z-Value | p-Value |
|---|---|---|---|---|---|---|
| Lowland tropical | Chilo partellus | 17.54 ± 2.81 a | 13.67 ± 2.52 a | 0.77 | −0.87 | 0.38 |
| Sesamia calamistis | 4.40 ± 0.86 b | 1.6 ± 0.28 a | 12.84 | 3.48 | 0.0003 | |
| Spodoptera frugiperda | 0.00 ± 0.00 a | 19.54 ± 2.44 b | 217.59 | −0.006 | <0.0001 | |
| Dry mid-altitude | Busseola fusca | 4.53 ± 0.75 a | 2.70 ± 0.56 a | 3.69 | 1.91 | 0.05 |
| Sesamia calamistis | 10.00 ± 1.74 a | 6.53 ± 0.92 a | 3.06 | 1.74 | 0.07 | |
| Chilo partellus | 22.80 ± 5.11 a | 14.11 ± 1.80 a | 3.70 | 1.92 | 0.05 | |
| Spodoptera frugiperda | 0.00 ± 0.00 a | 6.76 ± 1.44 b | 71.72 | −0.004 | <0.0001 | |
| Dry transitional | Busseola fusca | 28.94 ± 3.18 a | 23.41 ± 3.98 a | 1.32 | 1.15 | 0.24 |
| Sesamia calamistis | 4.80 ± 1.54 a | 3.73 ± 0.53 a | 0.3713 | 0.610 | 0.54 | |
| Chilo partellus | 1.67 ± 0.49 a | 2.20 ± 0.45 a | 0.553 | −743 | 0.45 | |
| Spodoptera frugiperda | 0.00 ± 0.00 a | 12.41 ± 2.34 b | 85.31 | −7.84 | <0.0001 | |
| Moist mid-altitude | Busseola fusca | 8.40 ± 2.18 a | 7.20 ± 0.86 a | 0.2301 | 0.480 | 0.63 |
| Sesamia calamistis | 17.15 ± 1.68 b | 11.52 ± 1.59 a | 5.94 | 2.44 | 0.01 | |
| Chilo partellus | 26.73 ± 2.84 b | 16.05 ± 1.90 a | 11.89 | 3.46 | 0.0005 | |
| Spodoptera frugiperda | 0.00 ± 0.00 a | 7.66 ± 1.15 b | 107.43 | 0.003 | <0.0001 | |
| Moist transitional | Busseola fusca | 9.80 ± 1.25 a | 9.86 ± 1.33 a | 0.0015 | −0.039 | 0.96 |
| Sesamia calamistis | 7.92 ± 1.56 a | 5.54 ± 1.42 a | 1.1178 | 1.065 | 0.29 | |
| Chilo partellus | 7.13 ± 1.12 a | 6.60 ± 1.09 a | 0.12 | 0.35 | 0.72 | |
| Spodoptera frugiperda | 0.00 ± 0.00 a | 10.80 ± 1.69 b | 122.11 | −0.003 | <0.0001 | |
| Highland tropical | Busseola fusca | 20.93 ± 2.76 a | 19.00 ± 2.70 a | 0.071 | 0.267 | 0.78 |
| Sesamia calamistis | 10.13 ± 1.24 a | 9.33 ± 0.82 a | 0.287 | 0.536 | 0.59 | |
| Spodoptera frugiperda | 0.00 ± 0.00 a | 3.33 ± 1.00 b | 26.698 | −0.004 | <0.0001 |
| AEZs | Species | Before FAW | After FAW | Total Number |
|---|---|---|---|---|
| Lowland tropical | Chilo partellus | 383 (63.31) | 386 (41.15) | 769 (49.84) |
| Sesamia calamistis | 222 (36.69) | 122 (13.01) | 344 (22.29) | |
| Spodoptera frugiperda | - | 430 (45.84) | 430 (27.87) | |
| Dry mid-altitude | Busseola fusca | 68 (12.14) | 46 (8.98) | 114 (10.63) |
| Sesamia calamistis | 150 (26.79) | 111 (21.68) | 261 (24.35) | |
| Chilo partellus | 342 (61.07) | 240 (46.88) | 582 (54.29) | |
| Spodoptera frugiperda | - | 115 (22.46) | 115 (10.73) | |
| Dry transitional | Busseola fusca | 550 (39.74) | 398 (36.92) | 948 (38.51) |
| Sesamia calamistis | 326 (23.55) | 196 (18.18) | 522 (21.20) | |
| Chilo partellus | 508 (36.71) | 273 (25.32) | 781 (31.72) | |
| Spodoptera frugiperda | - | 211 (19.57) | 211 (8.57) | |
| Moist mid-altitude | Busseola fusca | 126 (56.50) | 108 (34.29) | 234 (43.49) |
| Sesamia calamistis | 72 (32.44) | 56 (17.78) | 128 (23.79) | |
| Chilo partellus | 25 (11.21) | 33 (10.48) | 58 (10.78) | |
| Spodoptera frugiperda | - | 118 (37.46) | 118 (21.93) | |
| Moist transitional | Busseola fusca | 147 (45.94) | 148 (34.18) | 295 (39.18) |
| Sesamia calamistis | 66 (20.63) | 24 (5.54) | 90 (11.95) | |
| Chilo partellus | 107 (36.71) | 99 (22.86) | 206 (27.36) | |
| Spodoptera frugiperda | NA | 162 (37.41) | 162 (21.51) | |
| Highland tropical | Busseola fusca | 299 (66.30) | 285 (60.00) | 584 (63.07) |
| Sesamia calamistis | 152 (33.70) | 140 (29.47) | 292 (31.53) | |
| Spodoptera frugiperda | NA | 50 (10.53) | 50 (5.40) | |
| Total number | 3543 | 3751 | 7294 |
References
- Kfir, R.; Overholt, W.A.; Khan, Z.R.; Polaszek, A. Biology and management of economicaly important lepidopteran cereal stem borers in Africa. Annu. Rev. Entomol. 2002, 47, 701–731. [Google Scholar] [CrossRef]
- Van den Berg, J.; Van Rensburg, J.B.J.; Pringle, K.L. Comparative injuriousness of Busseola fusca (Lepidoptera: Noctuidae) and Chilo partellus (Lepidoptera: Pyralidae) on grain sorghum. Bull. Entomol. Res. 1991, 81, 137–142. [Google Scholar] [CrossRef]
- Tefera, T. Lepidopterous stem borers of sorghum and their natural enemies in eastern Ethiopia. Trop. Sci. 2004, 44, 128–130. [Google Scholar] [CrossRef]
- Ong’amo, G.; Le Ru, B.P.; Dupas, S.; Moyal, P.; Calatayud, P.-A.; Silvain, J.-F. Distribution, pest status and agro-climatic preferences of lepidopteran stem borers of maize in Kenya. Ann. Soc. Entomol. Fr. 2006, 42, 171–177. [Google Scholar] [CrossRef]
- Krüger, W.; van den Berg, J.; van Hamburg, H. The relative abundance of maize stem borers and their parasitoids at the Tshiombo irrigation scheme in Venda, South Africa. S. Afr. J. Plant Soil 2008, 25, 144–151. [Google Scholar] [CrossRef]
- Mwalusepo, S.; Tonnang, H.E.Z.; Massawe, E.S.; Okuku, G.O.; Khadioli, N.; Johansson, T.; Calatayud, P.-A.; Le Ru, B.P. Predicting the impact of temperature change on the future distribution of maize stem borers and their natural enemies along East African mountain gradients using phenology models. PLoS ONE 2015, 10, e0130427. [Google Scholar]
- Calatayud, P.-A.; Njuguna, E.; Mwalusepo, S.; Gathara, M.; Okuku, G.; Kibe, A.; Musyoka, B.; Williamson, D.; Ong’amo, G.; Juma, G.; et al. Can climate-driven change influence silicon assimilation by cereals and hence the distribution of lepidopteran stem borers in East Africa? Agric. Ecosyst. Environ. 2016, 224, 95–103. [Google Scholar] [CrossRef]
- Ntiri, E.S.; Calatayud, P.-A.; Van Den Berg, J.; Le Ru, B.P. Spatio-temporal interactions between maize lepidopteran stemborer communities and possible implications from the recent invasion of Spodoptera frugiperda (Lepidoptera: Noctuidae) in Sub-Saharan Africa. Environ. Entomol. 2019, 48, 573–582. [Google Scholar] [CrossRef] [PubMed]
- Guofa, Z.; Overholt, W.A.; Mochiah, M.B. Changes in the distribution of lepidopteran maize stemborers in Kenya from the 1950s to 1990s. Int. J. Trop. Insect Sci. 2001, 21, 395–402. [Google Scholar] [CrossRef] [Green Version]
- Ong’amo, G.; Le Ru, B.; Dupas, S.; Moyal, P.; Muchugu, E.; Calatayud, P.-A.; Silvain, J.-F. The role of wild host plants in the abundance of lepidopteran stem borers along altitudinal gradient in Kenya. Ann. Soc. Entomol. Fr. 2006, 42, 363–370. [Google Scholar] [CrossRef] [Green Version]
- Ntiri, E.S.; Calatayud, P.-A.; Van den Berg, J.; Le Ru, B.P. Density dependence and temporal plasticity of competitive interactions during utilisation of resources by a community of lepidopteran stemborer species. Entomol. Exp. Appl. 2017, 162, 272–283. [Google Scholar] [CrossRef]
- Ntiri, E.S.; Calatayud, P.-A.; Van Den Berg, J.; Schulthess, F.; Le Ru, B.P. Influence of temperature on intra- and interspecific resource utilization within a community of lepidopteran maize stemborers. PLoS ONE 2016, 11, e148735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mailafiya, D.M.; Le Ru, B.P.; Kairu, E.W.; Calatayud, P.-A.; Dupas, S. Species diversity of lepidopteran stem borer parasitoids in cultivated and natural habitats in Kenya. J. Appl. Entomol. 2009, 133, 416–429. [Google Scholar] [CrossRef]
- Mailafiya, D.M.; Le Ru, B.P.; Kairu, E.W.; Calatayud, P.-A.; Dupas, S. Factors affecting stem borer parasitoid species diversity and parasitism in cultivated and natural habitats. Environ. Entomol. 2010, 39, 57–67. [Google Scholar] [CrossRef] [Green Version]
- Mailafiya, D.M.; Le Ru, B.P.; Kairu, W.E.; Calatayud, P.-A.; Dupas, S. Geographic distribution, host range and perennation of Cotesia sesamiae and Cotesia flavipes Cameron in cultivated and natural habitats in Kenya. Biol. Control 2010, 54, 1–8. [Google Scholar] [CrossRef]
- Mailafiya, D.M.; Le Ru, B.P.; Kairu, E.W.; Dupas, S.; Calatayud, P.-A. Parasitism of Lepidopterous stem borers in cultivated and natural habitats. J. Insect Sci. 2011, 11, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Overholt, W.; Ngi-Song, A.J.; Mbapila, J.; Lammers, P.; Kioko, E. Ecological considerations of the introduction of Cotesia flavipes Cameron (Hymenoptera: Braconidae) for biological control of Chilo partellus (Lepidoptera: Pyralidae) in Africa. Biocontrol News Inf. 1994, 15, 19N–24N. [Google Scholar]
- Overholt, W.A.; Ngi-Song, A.J.; Omwega, C.O.; Kimani-Njogu, S.W.; Mbapila, J.; Sallam, M.N.; Ofomata, V. A review of the introduction and establishment of Cotesia flavipes Cameron in East Africa for biological control of cereal stem borers. Insect Sci. Appl. 1997, 17, 79–88. [Google Scholar]
- Sokame, B.M.; Rebaudo, F.; Musyoka, B.; Obonyo, J.; Mailafiya, D.M.; Le Ru, B.P.; Kilalo, C.D.; Juma, G.; Calatayud, P.-A. Carry-over niches for lepidopteran maize stemborers and associated parasitoids during non-cropping season. Insect 2019, 10, 191. [Google Scholar] [CrossRef] [Green Version]
- Songa, J.M.; Overholt, W.A.; Okello, R.O.; Mueke, J.M. Control of lepidopteran stemborers in maize by indigenous parasitoids in semi-arid areas of eastern Kenya. Biol. Agric. Hortic. 2002, 20, 77–90. [Google Scholar] [CrossRef]
- Goergen, G.; Kumar, P.L.; Sankung, S.B.; Togola, A.; Tamò, M. First report of outbreaks of the fall armyworm Spodoptera frugiperda (J E Smith) (Lepidoptera, Noctuidae), a new alien invasive pest in West and Central Africa. PLoS ONE 2016, 11, e0165632. [Google Scholar] [CrossRef] [Green Version]
- Rwomushana, I.; Bateman, M.; Beale, T.; Beseh, P.; Cameron, K.; Chiluba, M.; Clottey, V.; Davis, T.; Day, R.; Early, R.; et al. Fall Armyworm: Impacts and Implications for Africa; Evidence Note Update, October 2018; CABI: Wallingford, UK, 2018. [Google Scholar]
- Baudron, F.; Zaman-Allah, M.A.; Chaipa, I.; Chari, N.; Chinwada, P. Understanding the factors influencing fall armyworm (Spodoptera frugiperda J.E. Smith) damage in African smallholder maize fields and quantifying its impacts on yield. A case study in Eastern Zimbabwe. Crop Prot. 2019, 120, 141–150. [Google Scholar] [CrossRef]
- Kassie, M.; Wossen, T.; De Groote, H.; Tefera, T.; Subramanian, S.; Balew, S. Economic impacts of fall armyworm and its management strategies: Evidence from southern Ethiopia. Eur. Rev. Agric. Econ. 2020, 1–29. [Google Scholar] [CrossRef]
- De Groote, H.; Kimenju, S.C.; Munyua, B.; Palmas, S.; Kassie, M.; Bruce, A. Spread and impact of fall armyworm (Spodoptera frugiperda J. E. Smith) in maize production areas of Kenya. Agric. Ecosyst. Environ. 2020, 292, 106804. [Google Scholar] [CrossRef]
- Sisay, B.; Simiyu, J.; Malusi, P.; Likhayo, P.; Mendesil, E.; Elibariki, N.; Wakgar, M.; Ayalew, G.; Tefera, T. First report of the fall armyworm, Spodoptera frugiperda (Lepidoptera: Noctuidae), natural enemies from Africa. J. Appl. Entomol. 2018, 142, 800–804. [Google Scholar] [CrossRef]
- Morrill, W.L.; Greene, G.L. Distribution of Fall Armyworm larvae. 1. Regions of field corn plants infested by larvae. Environ. Entomol. 1973, 2, 195–198. [Google Scholar] [CrossRef]
- Van den Berg, J. Economy of Stem Borer Control in Sorghum; Crop protection Series no 2; ARC: Potchefstroom, South Africa, 1997. [Google Scholar]
- CAB International. How to Identify Fall Armyworm. Poster. Plantwise. 2017. Available online: https://www.cabi.org/isc/fallarmyworm (accessed on 25 May 2021).
- Sokame, B.M. Functioning of a Community of Lepidopteran Maize Stemborers and Associated Parasitoids in the Context of the Recent Invasion of the Fall Armyworm in Kenya. Ph.D. Thesis, University of Nairobi, Nairobi, Kenya, 26 November 2020. [Google Scholar]
- Hassan, R.M.; Corbett, J.D.; Njoroge, K. Combining geo-referenced survey data with agroclimatic attributes to characterize maize production systems in Kenya. In Maize Technology Development and Transfer: A GIS Application for Research Planning in Kenya; Hassan, R.M., Ed.; CAB International: Wallington, WA, USA; Oxon, UK, 1998; pp. 43–68. [Google Scholar]
- Overholt, W.; Ogedah, K.; Lammers, P. Distribution and sampling of Chilo partellus (Lepidoptera: Pyralidae) in maize and sorghum on the Kenya coast. Bull. Entomol. Res. 1994, 84, 367–378. [Google Scholar] [CrossRef]
- Onyango, F.O.; Ochieng’-Odero, J.E.R. Continuous rearing of the maize stem borer Busseola fusca on an artificial diet. Entomol. Exp. Appl. 1994, 73, 139–144. [Google Scholar] [CrossRef]
- Prasanna, B.M.; Bruce, A.; Winter, S.; Otim, M.; Asea, G.; Subramanian, S.; Malick, B.M.; van den Berg, J.; Beiriger, R.; Gichuru, L.; et al. Host Plant Resistance to Fall Armyworm. In Fall Armyworm in Africa: A Guide for Integrated Pest Management a Guide for Integrated Pest Management, 1st ed.; Prasanna, B.M., Huesing, J.E., Eddy, R., Peschke, V.M., Eds.; USAID & CIMMYT, CGIAR and Research Program on Maize, Mexico: Mexico City, Mexico, 2018; pp. 45–62. [Google Scholar]
- Polaszek, A.; Walker, A.K. The Cotesia flavipes species-complex. Parasitoids of cereal stem borers in the tropics. Redia 1991, 74, 335–341. [Google Scholar]
- Kimani-Njogu, S.W.; Overholt, W.A. Biosystematics of the Cotesia flavipes species complex (Hymenoptera: Braconidae), parasitoids of the gramineous stemborers. Insect Sci. Appl. 1997, 17, 119–130. [Google Scholar] [CrossRef]
- Zheng, X.-L.; Li, J.; Su, L.; Liu, J.-Y.; Meng, L.-Y.; Lin, M.-Y.; Zhang, J.; Lu, W. Ecological and morphological characteristics of parasitoids in Phauda flammans (Lepidoptera: Zygaenidae). Parasite 2015, 22, 36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting linear mixedeffects models using lme4. J. Stat. Soft 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Mangiafico, S.S. Summary and Analysis of Extension Program Evaluation in R; Rutgers Cooperative Extension: New Brunswick, NJ, USA, 2016; p. 775. [Google Scholar]
- Russell, V.L. Least-Squares Means: The R Package lsmeans. J. Stat. Soft 2016, 69, 1–33. [Google Scholar]
- Ndjomatchoua, F.T.; Tonnang, H.E.Z.; Plantamp, C.; Campagne, P.; Tchawoua, C.; Le Ru, B.P. Spatial and temporal spread of maize stem borer Busseola fusca (Fuller) (Lepidoptera: Noctuidae) damage in smallholder farms. Agric. Ecosyst. Environ. 2016, 235, 105–118. [Google Scholar] [CrossRef]
- Sokame, B.M.; Ntiri, S.E.; Ahuya, P.; Baldwyn, T.; Le Ru, B.P.; Kilalo, C.D.; Juma, G.; Calatayud, P.-A. Caterpillar-induced plant volatiles attract conspecific and heterospecific adults for oviposition within a community of lepidopteran stemborers on maize plant. Chemoecology 2019, 29, 89–101. [Google Scholar] [CrossRef]
- R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. Available online: https://www.r-project.org/ (accessed on 29 February 2020).
- Rejmanek, M.; Richardson, D.M. What attributes make some plant species more invasive? Ecology 1996, 77, 1655–1661. [Google Scholar] [CrossRef]
- Thébaud, C.; Finzi, A.C.; Affre, L.; Debussche, M.; Escarre, J. Assessing why two introduced conyza differ in their ability to invade mediterranean old fields. Ecology 1996, 77, 791–804. [Google Scholar] [CrossRef]
- Kolar, C.S.; Lodge, D.M. Progress in invasion biology: Predicting invaders. Trends Ecol. Evol. 2001, 16, 199–204. [Google Scholar] [CrossRef]
- Hailu, G.; Niassy, S.; Bässler, T.; Ochatum, N.; Studer, C.; Salifu, D.; Agbodzavu, M.K.; Khan, Z.R.; Midega, C.; Subramanian, S. Could fall armyworm, Spodoptera frugiperda (J. E. Smith) invasion in Africa contribute to the displacement of cereal stemborers in maize and sorghum cropping systems. Int. J. Trop. Insect Sci. 2021. [Google Scholar] [CrossRef]
- Sokame, B.M.; Tonnang, H.E.Z.; Subramanian, S.; Bruce, A.Y.; Dubois, T.; Ekesi, S.; Calatayud, P.-A. A system dynamic model for pests and natural enemies interactions. Sci. Rep. 2021, 11, 1401. [Google Scholar] [CrossRef]
- Boukal, D.; Kivan, V. Lyapunov functions for Lotka-Volterra predator-prey models with optimal foraging behavior. J. Math. Biol. 1999, 39, 493–517. [Google Scholar] [CrossRef] [Green Version]
- Sokame, B.M.; Obonyo, J.; Sammy, E.M.; Mohamed, S.A.; Subramanian, S.; Kilalo, C.D.; Juma, G.; Calatayud, P.-A. Impact of the exotic fall armyworm on larval parasitoids associated with the lepidopteran maize stemborers in Kenya. BioControl 2020, 66, 193–204. [Google Scholar] [CrossRef]
- Chabaane, Y.; Laplanche, D.; Turlings, T.C.; Desurmont, G.A. Impact of exotic insect herbivores on native tritrophic interactions: A case study of the African cotton leafworm, Spodoptera littoralis and insects associated with the field mustard Brassica rapa. J. Ecol. 2015, 103, 109–117. [Google Scholar] [CrossRef]
- Desurmont, G.A.; Harvey, J.; van Dam, N.M.; Cristescu, S.M.; Schiestl, F.P.; Cozzolino, S.; Anderson, P.; Larsson, M.C.; Kindlmann, P.; Danner, H.; et al. Alien interferences: Disruption of infochemical networks by invasive insect herbivores. Plant Cell Environ. 2014, 37, 1854–1865. [Google Scholar] [CrossRef] [PubMed]
- Abram, P.K.; Gariepy, T.D.; Boivin, G.; Brodeur, J. An invasive stink bug as an evolutionary trap for an indigenous egg parasitoid. Biol. Invasions 2014, 16, 1387–1395. [Google Scholar] [CrossRef]
- Hernandez-Mendoza, J.; Lopez-Barbosa, E.; Garza-Gonzalez, E.; Mayek-Perez, N. Spatial distribution of Spodoptera frugiperda (Lepidoptera: Noctuidae) in maize landraces grown in Colima, Mexico. Int. J. Trop. Insect Sci. 2008, 28, 126–129. [Google Scholar] [CrossRef]
- Wyckhuys, K.A.G.; O’Neil, R.J. Population dynamics of Spodoptera frugiperda Smith (Lepidoptera: Noctuidae) and associated arthropod natural enemies in Honduran subsistence maize. Crop Prot. 2006, 25, 1180–1190. [Google Scholar] [CrossRef]






| Maize Pests | Parasitoid Species | Agro-Ecological Zones | |||||
|---|---|---|---|---|---|---|---|
| Lowland Tropical | Dry Mid-Altitude | Dry Transitional | Moist Transitional | Moist Mid-Altitude | Highland Tropical | ||
| Chilo partellus | Hymenoptera: Braconidae | ||||||
| Cotesia flavipes | x | x | x | x | x | ||
| Cotesia sesamiae | x | ||||||
| Chelonus curvimaculatus | x | ||||||
| Hymenoptera: Ichneumonidae | |||||||
| Xanthopimpla stemmator | x | ||||||
| Pediobius furvus | x | ||||||
| Hymenoptera: Ceraphronidae | |||||||
| Aphanogmus fijiensis | x | ||||||
| Sesamia calamistis | Hymenoptera: Braconidae | ||||||
| Cotesia flavipes | x | x | |||||
| Cotesia sesamiae | x | x | x | x | x | x | |
| Habrobracon sp. | x | ||||||
| Dolichoginedea polaszeki | x | x | |||||
| Diptera: Tachinidae | |||||||
| Siphona murina | x | x | |||||
| Descampsina sesamiae | x | ||||||
| Busseola fusca | Hymenoptera: Braconidae | ||||||
| Cotesia sesamiae | x | x | x | ||||
| Dolichoginedea polaszeki | x | ||||||
| Diptera: Tachinidae | |||||||
| Siphona murina | x | x | |||||
| Sturmiopsis parasitica | x | x | x | ||||
| Hymenoptera: Ichneumonidae | |||||||
| Xanthopimpla stemmator | x | ||||||
| Spodoptera frugiperda | Hymenoptera: Braconidae | ||||||
| Habrobracon sp. | x | x | |||||
| Diptera: Tachinidae | |||||||
| Sturmiopsis parasitica | x | ||||||
| Palexorista zonata | x | ||||||
| Hymenoptera: Ichneumonidae | |||||||
| Charops ater | x | x | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sokame, B.M.; Musyoka, B.; Obonyo, J.; Rebaudo, F.; Abdel-Rahman, E.M.; Subramanian, S.; Kilalo, D.C.; Juma, G.; Calatayud, P.-A. Impact of an Exotic Invasive Pest, Spodoptera frugiperda (Lepidoptera: Noctuidae), on Resident Communities of Pest and Natural Enemies in Maize Fields in Kenya. Agronomy 2021, 11, 1074. https://doi.org/10.3390/agronomy11061074
Sokame BM, Musyoka B, Obonyo J, Rebaudo F, Abdel-Rahman EM, Subramanian S, Kilalo DC, Juma G, Calatayud P-A. Impact of an Exotic Invasive Pest, Spodoptera frugiperda (Lepidoptera: Noctuidae), on Resident Communities of Pest and Natural Enemies in Maize Fields in Kenya. Agronomy. 2021; 11(6):1074. https://doi.org/10.3390/agronomy11061074
Chicago/Turabian StyleSokame, Bonoukpoè Mawuko, Boaz Musyoka, Julius Obonyo, François Rebaudo, Elfatih M. Abdel-Rahman, Sevgan Subramanian, Dora Chao Kilalo, Gérald Juma, and Paul-André Calatayud. 2021. "Impact of an Exotic Invasive Pest, Spodoptera frugiperda (Lepidoptera: Noctuidae), on Resident Communities of Pest and Natural Enemies in Maize Fields in Kenya" Agronomy 11, no. 6: 1074. https://doi.org/10.3390/agronomy11061074