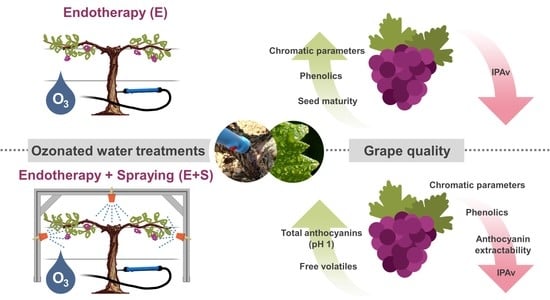
Novel Endotherapy-Based Applications of Ozonated Water to Bobal Grapevines: Effect on Grape Quality
Abstract
:1. Introduction
2. Materials and Methods
2.1. Grapevines
2.2. Ozonated Water
2.3. Grapevine Treatments
2.4. Analytical Methods
2.4.1. Sample Preparation
2.4.2. Grape Enological Parameters
2.4.3. Chromatic Parameters
2.4.4. Phenolic Maturity
2.4.5. Varietal Aroma Potential Index (IPAv)
2.4.6. Determination of Low Molecular Weight Phenolic Compounds by HPLC-DAD
2.4.7. Determination of Volatile Compounds by HS-SBSE-GC-MS
2.5. Statistical Analysis
3. Results and Discussion
3.1. Effect on Enological Parameters
3.2. Effect on Chromatic Parameters
3.3. Effect on Phenolic Maturity
3.4. Effect on Phenolic Compounds
3.5. Effect on the Varietal Aroma Potential Index (IPAv)
3.6. Effect on Volatile Compounds
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Compant, S.; Brader, G.; Muzammil, S.; Sessitsch, A.; Lebrihi, A.; Mathieu, F. Use of beneficial bacteria and their secondary metabolites to control grapevine pathogen diseases. BioControl 2013, 58, 435–455. [Google Scholar] [CrossRef] [Green Version]
- Martelli, G.P. Infectious diseases and certification of grapevines. Options Méditerranéennes Série B 1997, 29, 47–64. [Google Scholar]
- Gramaje, D.; Urbez-Torres, J.R.; Sosnowski, M.R. Managing grapevine trunk diseases with respect to etiology and epidemiology: Current strategies and future prospects. Plant Dis. 2018, 102, 12–39. [Google Scholar] [CrossRef] [Green Version]
- Bertsch, C.; Ramírez-Suero, M.; Magnin-Robert, M.; Larignon, P.; Chong, J.; Abou-Mansour, E.; Spagnolo, A.; Clément, C.; Fontaine, F. Grapevine trunk diseases: Complex and still poorly understood. Plant Pathol. 2013, 62, 243–265. [Google Scholar] [CrossRef] [Green Version]
- Florence, F.; Gramaje, D.; Armengol, J.; Smart, R.; Nagy, Z.A.; Borgo, M.; Rego, C.; Corio-costet, M.F. Grapevine Trunk Diseases. A review, 1st ed.; OIV Publications: Paris, France, 2016. [Google Scholar]
- Pierron, R.J.G.; Pages, M.; Couderc, C.; Compant, S.; Jacques, A.; Violleau, F. In vitro and in planta fungicide properties of ozonated water against the esca-associated fungus Phaeoacremonium aleophilum. Sci. Hortic. 2015, 189, 184–191. [Google Scholar] [CrossRef] [Green Version]
- Campayo, A.; Serrano de la Hoz, K.; García-Martínez, M.M.; Sánchez-Martínez, J.F.; Salinas, M.R.; Alonso, G.L. Spraying ozonated water on Bobal grapevines: Effect on grape quality. Food Res. Int. 2019, 125, 108540. [Google Scholar] [CrossRef]
- Modesti, M.; Baccelloni, S.; Brizzolara, S.; Aleandri, M.P.; Bellincontro, A.; Mencarelli, F.; Tonutti, P. Effects of treatments with ozonated water in the vineyard (cv Vermentino) on microbial population and fruit quality parameters. BIO Web Conf. 2019, 13, 04011. [Google Scholar] [CrossRef]
- Hoigné, J. Chemistry of aqueous ozone and transformation of pollutants by ozonation and advanced oxidation processes. In Quality and Treatment of Drinking Water II. The Handbook of Environmental Chemistry (Part C: Water Pollution); Hrubec, J., Ed.; Springer: Berlin, Germany, 1998; Volume 5, pp. 83–141. [Google Scholar]
- Kim, J.G.; Yousef, A.E.; Dave, S. Application of ozone for enhancing the microbiological safety and quality of foods: A review. J. Food Prot. 1999, 62, 1071–1087. [Google Scholar] [CrossRef]
- Brodowska, A.J.; Nowak, A.; Śmigielski, K. Ozone in the food industry: Principles of ozone treatment, mechanisms of action, and applications: An overview. Crit. Rev. Food Sci. Nutr. 2018, 58, 2176–2201. [Google Scholar] [CrossRef]
- Khadre, M.A.; Yousef, A.E.; Kim, J.G. Microbiological aspects of ozone applications in food: A review. J. Food Sci. 2001, 66, 1242–1252. [Google Scholar] [CrossRef]
- Glowacz, M.; Colgan, R.; Rees, D. The use of ozone to extend the shelf-life and maintain quality of fresh produce. J. Sci. Food Agric. 2015, 95, 662–671. [Google Scholar] [CrossRef]
- Botondi, R.; De Sanctis, F.; Moscatelli, N.; Vettraino, A.M.; Catelli, C.; Mencarelli, F. Ozone fumigation for safety and quality of wine grapes in postharvest dehydration. Food Chem. 2015, 188, 641–647. [Google Scholar] [CrossRef]
- Bellincontro, A.; Catelli, C.; Cotarella, R.; Mencarelli, F. Postharvest ozone fumigation of Petit Verdot grapes to prevent the use of sulfites and to increase anthocyanin in wine. Aust. J. Grape Wine Res. 2017, 23, 200–206. [Google Scholar] [CrossRef]
- De Sanctis, F.; Ceccantoni, B.; Bellincontro, A.; Botondi, R.; Mencarelli, F.; D’Onofrio, C.; Ducci, E.; Catelli, C. Ozone fumigation postharvest treatment for the quality of wine grape. ISHS Acta Hortic. 2015, 1071, 795–800. [Google Scholar] [CrossRef]
- Río Segade, S.; Vilanova, M.; Giacosa, S.; Perrone, I.; Chitarra, W.; Pollon, M.; Torchio, F.; Boccacci, P.; Gambino, G.; Gerbi, V.; et al. Ozone improves the aromatic fingerprint of white grapes. Sci. Rep. 2017, 7, 16301. [Google Scholar] [CrossRef] [Green Version]
- Guzzon, R.; Franciosi, E.; Moser, S.; Carafa, I.; Larcher, R. Application of ozone during grape drying for the production of straw wine. Effects on the microbiota and compositive profile of grapes. J. Appl. Microbiol. 2018, 125, 513–527. [Google Scholar] [CrossRef]
- Carbone, K.; Mencarelli, F. Influence of short-term postharvest ozone treatments in nitrogen or air atmosphere on the metabolic response of white wine grapes. Food Bioprocess Technol. 2015, 8, 1739–1749. [Google Scholar] [CrossRef]
- Río Segade, S.; Vilanova, M.; Pollon, M.; Giacosa, S.; Torchio, F.; Rolle, L. Grape VOCs response to postharvest short-term ozone treatments. Front. Plant Sci. 2018, 9, 1826. [Google Scholar] [CrossRef]
- Graham, T.; Zhang, P.; Woyzbun, E.; Dixon, M. Response of hydroponic tomato to daily applications of aqueous ozone via drip irrigation. Sci. Hortic. 2011, 129, 464–471. [Google Scholar] [CrossRef]
- Fujiwara, K.; Fujii, T. Effects of spraying ozonated water on the severity of powdery mildew infection on cucumber leaves. Ozone Sci. Eng. 2002, 24, 463–469. [Google Scholar] [CrossRef]
- Campayo, A.; Serrano de la Hoz, K.; García-Martínez, M.M.; Salinas, M.R.; Alonso, G.L. Spraying ozonated water on Bobal grapevines: Effect on wine quality. Biomolecules 2020, 10, 213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forney, C.F. Postharvest response of horticultural products to ozone. In Postharvest Oxidative Stress in Horticultural Crops; Hodges, D.M., Ed.; Food Products Press: New York, NY, USA, 2003; pp. 13–43. [Google Scholar]
- Sandermann, H.; Ernst, D.; Heller, W.; Langebartels, C. Ozone: An abiotic elicitor of plant defence reactions. Trends Plant Sci. 1998, 3, 47–50. [Google Scholar] [CrossRef]
- Heath, R.L. Modification of the biochemical pathways of plants induced by ozone: What are the varied routes to change? Environ. Pollut. 2008, 155, 453–463. [Google Scholar] [CrossRef] [PubMed]
- Booker, F.L.; Miller, J.E. Phenylpropanoid metabolism and phenolic composition of soybean [Glycine max (L.) Merr.] leaves following exposure to ozone. J. Exp. Bot. 1998, 49, 1191–1202. [Google Scholar] [CrossRef]
- Beauchamp, J.; Wisthaler, A.; Hansel, A.; Kleist, E.; Miebach, M.; Niinemets, Ü.; Schurr, U.; Wildt, J. Ozone induced emissions of biogenic VOC from tobacco: Relationships between ozone uptake and emission of LOX products. Plant Cell Environ. 2005, 28, 1334–1343. [Google Scholar] [CrossRef]
- Loreto, F.; Pinelli, P.; Manes, F.; Kollist, H. Impact of ozone on monoterpene emissions and evidence for an isoprene-like antioxidant action of monoterpenes emitted by Quercus ilex leaves. Tree Physiol. 2004, 24, 361–367. [Google Scholar] [CrossRef]
- Pinelli, P.; Tricoli, D. A new approach to ozone plant fumigation: The Web-O3-Fumigation. Isoprene response to a gradient of ozone stress in leaves of Quercus pubescens. iForest 2008, 1, 22–26. [Google Scholar] [CrossRef] [Green Version]
- Bonicelli, B.; Naud, O.; Rousset, S.; Sinfort, C.; de Rudnicki, V.; Lescot, J.M.; Ruelle, L.; Scheyer, L.; Cotteux, E. The challenge for precision spraying. In Proceedings of the AgEng 2010: International Conference on Agricultural Engineering, Clermont-Ferrand, France, 6–8 September 2010; p. 11. [Google Scholar]
- Berger, C.; Laurent, F. Trunk injection of plant protection products to protect trees from pests and diseases. Crop Prot. 2019, 124, 104831. [Google Scholar] [CrossRef]
- Montecchio, L. A venturi effect can help cure our trees. J. Vis. Exp. 2013, 80, e51199. [Google Scholar] [CrossRef] [Green Version]
- Di Marco, S.; Mazzullo, A.; Calzarano, F.; Cesari, A. The control of esca: Status and perspectives. Phytopathol. Mediterr. 2000, 39, 232–240. [Google Scholar]
- Calzarano, F.; Di Marco, S.; Cesari, A. Benefit of fungicide treatment after trunk renewal of vines with different types of esca necrosis. Phytopathol. Mediterr. 2004, 43, 116–124. [Google Scholar]
- Darrieutort, G.; Lecomte, P. Evaluation of a trunk injection technique to control grapevine wood diseases. Phytopathol. Mediterr. 2007, 46, 50–57. [Google Scholar]
- Dula, T.; Kappes, E.M.; Horvath, A.; Rabai, A. Preliminary trials on treatment of esca-infected grapevines with trunk injection of fungicides. Phytopathol. Mediterr. 2007, 46, 91–95. [Google Scholar]
- Del Frari, G.; Costa, J.; Oliveira, H.; Boavida Ferreira, R. Endotherapy of infected grapevine cuttings for the control of Phaeomoniella chlamydospora and Phaeoacremonium minimum. Phytopathol. Mediterr. 2018, 57, 439–448. [Google Scholar]
- Düker, A.; Kubiak, R. Stem application of metalaxyl for the protection of Vitis vinifera L. (‘Riesling’) leaves and grapes against downy mildew (Plasmopara viticola). Vitis J. Grapevine Res. 2009, 48, 43–48. [Google Scholar]
- Coombe, B.G. Growth Stages of the Grapevine: Adoption of a system for identifying grapevine growth stages. Aust. J. Grape Wine Res. 1995, 1, 104–110. [Google Scholar] [CrossRef]
- EU Official Methods for Wine Analyses, Regulation 440/2003; European Commission: Brussels, Belgium, 2003.
- Glories, Y. La couleur des vins rouges. Les equilibres des anthocyanes et des tanins. Connaiss. Vigne Vin. 1984, 18, 195–217. [Google Scholar] [CrossRef] [Green Version]
- Saint-Cricq de Gaulejac, N.; Vivas, N.; Glories, Y. Maturité phénolique: Définition et contrôle. Revue Française d’Oenologie 1998, 173, 22–25. [Google Scholar]
- Ribéreau-Gayon, J.; Peynaud, E.; Sudraud, P.; Ribéreau-Gayon, P. Traité d’Oenologie-Sciences et Techniques du vin, Tome I: Analyse et Contrôle Des Vins; Dunod: Paris, France, 1982. [Google Scholar]
- Puissant, A.; Léon, H. La matière colorante des grains de raisins de certains cépages cultivés en Anjou en 1965. Ann. Technol. Agric. 1967, 16, 217–225. [Google Scholar]
- Salinas, M.R.; Serrano De La Hoz, K.; Zalacain, A.; Lara, J.F.; Garde-Cerdán, T. Analysis of red grape glycosidic aroma precursors by glycosyl glucose quantification. Talanta 2012, 89, 396–400. [Google Scholar] [CrossRef]
- Pardo-García, A.I.; Martínez-Gil, A.M.; Cadahía, E.; Pardo, F.; Alonso, G.L.; Salinas, M.R. Oak extract application to grapevines as a plant biostimulant to increase wine polyphenols. Food Res. Int. 2014, 55, 150–160. [Google Scholar] [CrossRef]
- Chen, C.P.; Frank, T.D.; Long, S.P. Is a short, sharp shock equivalent to long-term punishment? Contrasting the spatial pattern of acute and chronic ozone damage to soybean leaves via chlorophyll fluorescence imaging. Plant Cell Environ. 2009, 32, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Artés-Hernández, F.; Aguayo, E.; Artés, F.; Tomás-Barberán, F.A. Enriched ozone atmosphere enhances bioactive phenolics in seedless table grapes after prolonged shelf life. J. Sci. Food Agric. 2007, 87, 824–831. [Google Scholar] [CrossRef]
- Rodoni, L.; Casadei, N.; Concellón, A.; Chaves Alicia, A.R.; Vicente, A.R. Effect of short-term ozone treatments on tomato (Solanum lycopersicum L.) fruit quality and cell wall degradation. J. Agric. Food Chem. 2010, 58, 594–599. [Google Scholar] [CrossRef] [PubMed]
- Tingey, D.T.; Fites, R.C.; Wickliff, C. Activity changes in selected enzymes from soybean leaves following ozone exposure. Physiol. Plant. 1975, 33, 316–320. [Google Scholar] [CrossRef]
- Laureano, J.; Giacosa, S.; Río Segade, S.; Torchio, F.; Cravero, F.; Gerbi, V.; Englezos, V.; Carboni, C.; Cocolin, L.; Rantsiou, K.; et al. Effects of continuous exposure to ozone gas and electrolyzed water on the skin hardness of table and wine grape varieties. J. Texture Stud. 2016, 47, 40–48. [Google Scholar] [CrossRef]
- D’Haese, D.; Horemans, N.; De Coen, W.; Guisez, Y. Identification of late O3-responsive genes in Arabidopsis thaliana by cDNA microarray analysis. Physiol. Plant. 2006, 128, 70–79. [Google Scholar] [CrossRef]
- Río Segade, S.; Paissoni, M.A.; Giacosa, S.; Bautista-Ortín, A.B.; Gómez-Plaza, E.; Gerbi, V.; Rolle, L. Winegrapes dehydration under ozone-enriched atmosphere: Influence on berry skin phenols release, cell wall composition and mechanical properties. Food Chem. 2019, 271, 673–684. [Google Scholar] [CrossRef]
- Paissoni, M.A.; Río Segade, S.; Giacosa, S.; Torchio, F.; Cravero, F.; Englezos, V.; Rantsiou, K.; Carboni, C.; Gerbi, V.; Teissedre, P.L.; et al. Impact of post-harvest ozone treatments on the skin phenolic extractability of red winegrapes cv Barbera and Nebbiolo (Vitis vinifera L.). Food Res. Int. 2017, 98, 68–78. [Google Scholar] [CrossRef]
- Pascual, O.; González-Royo, E.; Gil, M.; Gómez-Alonso, S.; García-Romero, E.; Canals, J.M.; Hermosín-Gutíerrez, I.; Zamora, F. Influence of grape seeds and stems on wine composition and astringency. J. Agric. Food Chem. 2016, 64, 6555–6566. [Google Scholar] [CrossRef]
- Cejudo-Bastante, M.J.; Vicario, A.; Guillén, D.A.; Hermosín-Gutiérrez, I.; Pérez-Coello, M.S. Phenolic characterization of minor red grape varieties grown in Castilla-La Mancha region in different vinification stages. Eur. Food Res. Technol. 2015, 240, 595–607. [Google Scholar] [CrossRef]
- Tiwari, B.K.; O’Donnell, C.P.; Patras, A.; Brunton, N.; Cullen, P.J. Anthocyanins and color degradation in ozonated grape juice. Food Chem. Toxicol. 2009, 47, 2824–2829. [Google Scholar] [CrossRef] [PubMed]
- Graham, T.; Zhang, P.; Zheng, Y.; Dixon, M.A. Phytotoxicity of aqueous ozone on five grown nursery species. HortScience 2009, 44, 774–780. [Google Scholar] [CrossRef]
- Rizzini, F.M.; Bonghi, C.; Tonutti, P. Postharvest water loss induces marked changes in transcript profiling in skins of wine grape berries. Postharvest Biol. Technol. 2009, 52, 247–253. [Google Scholar] [CrossRef]
- Pan, G.Y.; Chen, C.L.; Gratzl, J.S.; Chang, H. Model compound studies on the cleavage of glycosidic bonds by ozone in aqueous solution. Res. Chem. Intermed. 1995, 21, 205–222. [Google Scholar] [CrossRef]
- Fares, S.; Oksanen, E.; Lännenpää, M.; Julkunen-Tiitto, R.; Loreto, F. Volatile emissions and phenolic compound concentrations along a vertical profile of Populus nigra leaves exposed to realistic ozone concentrations. Photosynth. Res. 2010, 104, 61–74. [Google Scholar] [CrossRef] [PubMed]
- Mencarelli, F.; Bellincontro, A. Recent advances in postharvest technology of the wine grape to improve the wine aroma. J. Sci. Food Agric. 2018. [Google Scholar] [CrossRef]
- Pell, E.J.; Schlagnhaufer, C.D.; Arteca, R.N. Ozone-induced oxidative stress: Mechanisms of action and reaction. Physiol. Plant. 1997, 100, 264–273. [Google Scholar] [CrossRef]
- Sharkey, T.D.; Yeh, S. Isoprene emission from plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2001, 52, 407–436. [Google Scholar] [CrossRef]
- Vickers, C.E.; Gershenzon, J.; Lerdau, M.T.; Loreto, F. A unified mechanism of action for volatile isoprenoids in plant abiotic stress. Nat. Chem. Biol. 2009, 5, 283–291. [Google Scholar] [CrossRef]
- Loreto, F.; Mannozzi, M.; Maris, C.; Nascetti, P.; Ferranti, F.; Pasqualini, S. Ozone quenching properties of isoprene and its antioxidant role in leaves. Plant Physiol. 2001, 126, 993–1000. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loreto, F.; Schnitzler, J.P. Abiotic stresses and induced BVOCs. Trends Plant Sci. 2010, 15, 154–166. [Google Scholar] [CrossRef] [PubMed]
- Roufet, M.; Bayonove, C.L.; Cordonnier, R.E. Etude de la composition lipidique du raisin, Vitis vinifera L.: Evolution au cours de la maturation et localisation dans la baie. Vitis 1987, 26, 85–97. [Google Scholar]
- Wildt, J.; Kobel, K.; Schuh-Thomas, G.; Heiden, A.C. Emissions of oxygenated volatile organic compounds from plants part II: Emissions of saturated aldehydes. J. Atmos. Chem. 2003, 45, 173–196. [Google Scholar] [CrossRef]




| Treatments | C | E | E + S |
|---|---|---|---|
| Phenolic Acids (mg/L) | |||
| Gallic acid | 0.55 ± 0.04 b | 0.42 ± 0.01 a | 0.38 ± 0.01 a |
| Syringic acid | 1.51 ± 0.01 b | 1.58 ± 0.02 c | 1.45 ± 0.02 a |
| trans-Caftaric acid | 0.18 ± 0.00 b | 0.18 ± 0.00 b | 0.16 ± 0.00 a |
| trans-p-Coutaric acid | 0.19 ± 0.00 a | 0.19 ± 0.00 a | 0.19 ± 0.00 a |
| Σ Phenolic acids | 2.42 ± 0.03 b | 2.38 ± 0.01 b | 2.17 ± 0.01 a |
| Flavonols (mg/L) | |||
| Myricetin 3-O-glucuronide + glucoside 1 | 0.79 ± 0.03 a | 1.07 ± 0.05 b | 0.71 ± 0.01 a |
| Quercetin 3-O-glucuronide + glucoside 1 | 0.41 ± 0.01 a | 0.37 ± 0.01 a | 0.28 ± 0.06 a |
| Laricitrin 3-O-glucoside/galactoside 2 | nq 3 | 0.08 ± 0.00 | nq 3 |
| Syringetin 3-O-glucoside | 0.15 ± 0.01 a | 0.17 ± 0.02 a | 0.13 ± 0.01 a |
| Σ Flavonols | 1.35 ± 0.02 b | 1.69 ± 0.04 c | 1.12 ± 0.08 a |
| Anthocyanins (mg/L) | |||
| Delphinidin 3-O-glucoside | 1.99 ± 0.10 b | 2.72 ± 0.13 c | 1.48 ± 0.05 a |
| Cyanidin 3-O-glucoside | 1.68 ± 0.03 c | 1.38 ± 0.03 b | 0.93 ± 0.01 a |
| Petunidin 3-O-glucoside | 2.77 ± 0.10 b | 3.77 ± 0.08 c | 2.19 ± 0.08 a |
| Peonidin 3-O-glucoside | 20.06 ± 0.58 c | 18.52 ± 0.37 b | 14.70 ± 0.16 a |
| Malvidin 3-O-glucoside | 34.00 ± 1.24 a | 47.21 ± 0.99 b | 30.79 ± 0.12 a |
| Peonidin 3-O-(6′-acetyl)-glucoside | 0.84 ± 0.03 b | 0.88 ± 0.01 b | 0.74 ± 0.02 a |
| Malvidin 3-O-(6′-acetyl)-glucoside | 1.98 ± 0.07 a | 2.71 ± 0.04 b | 1.96 ± 0.03 a |
| Σ Anthocyanins | 63.32 ± 2.13 b | 77.18 ± 1.67 c | 52.78 ± 0.35 a |
| Treatments | C | E | E + S |
|---|---|---|---|
| C6 Compounds (µg/L) | |||
| 1-Hexanol | 315.87 ± 9.93 ab | 358.00 ± 15.16 b | 295.23 ± 13.88 a |
| cis-3-Hexen-1-ol | 14.32 ± 0.79 a | 14.27 ± 0.41 a | 13.61 ± 0.38 a |
| trans-2-Hexen-1-ol | 58.91 ± 1.20 a | 67.63 ± 2.38 a | 55.96 ± 5.23 a |
| trans-2-Hexenal | 14.45 ± 1.01 a | 19.29 ± 0.02 ab | 25.61 ± 3.04 b |
| Σ C6 compounds | 403.55 ± 10.53 a | 459.18 ± 17.98 a | 390.40 ± 21.78 a |
| Terpenoids (µg/L) | |||
| β-Damascenone | 0.06 ± 0.01 a | 0.16 ± 0.04 b | 0.06 ± 0.01 a |
| Geraniol | 2.95 ± 0.06 a | 3.31 ± 0.29 a | 2.99 ± 0.17 a |
| Geranyl acetone | 0.29 ± 0.04 a | 0.25 ± 0.03 a | 0.31 ± 0.07 a |
| Linalool | 0.40 ± 0.01 a | 0.35 ± 0.04 a | 0.61 ± 0.05 b |
| Σ Terpenoids | 3.69 ± 0.11 a | 4.08 ± 0.34 a | 3.96 ± 0.30 a |
| Volatile phenols (µg/L) | |||
| Benzaldehyde | 8.03 ± 1.11 a | 6.82 ± 0.70 a | 22.14 ± 3.11 b |
| Guaiacol | 7.94 ± 0.10 a | 7.74 ± 0.33 a | 12.97 ± 1.10 b |
| Syringol | 80.04 ± 7.00 a | 115.22 ± 3.56 a | 116.27 ± 27.55 a |
| Σ Volatile phenols | 96.02 ± 5.80 a | 129.78 ± 4.59 a | 151.38 ± 29.57 a |
| Others (µg/L) | |||
| Nonanal | 1.47 ± 0.11 a | 1.58 ± 0.08 a | 2.82 ± 0.29 b |
| 1-octen-3-ol | 1.22 ± 0.05 a | 1.31 ± 0.11 a | 1.15 ± 0.08 a |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campayo, A.; Serrano de la Hoz, K.; García-Martínez, M.M.; Salinas, M.R.; Alonso, G.L. Novel Endotherapy-Based Applications of Ozonated Water to Bobal Grapevines: Effect on Grape Quality. Agronomy 2020, 10, 1218. https://doi.org/10.3390/agronomy10091218
Campayo A, Serrano de la Hoz K, García-Martínez MM, Salinas MR, Alonso GL. Novel Endotherapy-Based Applications of Ozonated Water to Bobal Grapevines: Effect on Grape Quality. Agronomy. 2020; 10(9):1218. https://doi.org/10.3390/agronomy10091218
Chicago/Turabian StyleCampayo, Ana, Kortes Serrano de la Hoz, M. Mercedes García-Martínez, M. Rosario Salinas, and Gonzalo L. Alonso. 2020. "Novel Endotherapy-Based Applications of Ozonated Water to Bobal Grapevines: Effect on Grape Quality" Agronomy 10, no. 9: 1218. https://doi.org/10.3390/agronomy10091218