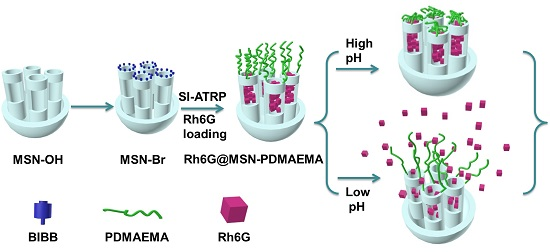
Surface Immobilization of pH-Responsive Polymer Brushes on Mesoporous Silica Nanoparticles by Enzyme Mimetic Catalytic ATRP for Controlled Cargo Release
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Measurements
2.3. Methods
2.3.1. ATRP-Initiator Immobilization (Synthesis of MSN-Br)
2.3.2. PDMAEMA Growth on MSNs by DhHP-6-Catalyzed MSN-Br SI-ATRP of DMAEMA
2.3.3. Rh6G Loading
2.3.4. Rh6G Release from Rh6G@MSN-PDMAEMA
3. Results and Discussion
3.1. ATRP-Initiator Immobilization
3.2. DhHP-6 Catalyzed Synthesis of MSN-PDMAEMA and Its Characterization
3.3. Rh6G Release from Rh6G@MSN-PDMAEMA in Solutions of Different pH Values
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kobayashi, S.; Makino, A. Enzymatic polymer synthesis: An opportunity for green polymer chemistry. Chem. Rev. 2009, 109, 5288–5353. [Google Scholar] [CrossRef] [PubMed]
- Hollmann, F.; Arends, I.W. Enzyme initiated radical polymerizations. Polymers 2012, 4, 759–793. [Google Scholar] [CrossRef]
- Singh, A.; Kaplan, D. In vitro Enzyme-induced vinyl polymerization enzyme-catalyzed synthesis of polymers. Adv. Polym. Sci. 2006, 194, 211–224. [Google Scholar]
- Hanoian, P.; Liu, C.T.; Hammes-Schiffer, S.; Benkovic, S. Perspectives on electrostatics and conformational motions in enzyme catalysis. Acc. Chem. Res. 2015, 48, 482–489. [Google Scholar] [CrossRef] [PubMed]
- Hamid, M.; Khalilur, R. Potential applications of peroxidases. Food Chem. 2009, 115, 1177–1186. [Google Scholar] [CrossRef]
- Xu, P.; Singh, A.; Kaplan, D. Enzymatic catalysis in the synthesis of polyanilines and derivatives of polyanilines enzyme-catalyzed synthesis of polymers. Adv. Polym. Sci. 2006, 194, 69–94. [Google Scholar]
- Kadokawa, J.; Kobayashi, S. Polymer synthesis by enzymatic catalysis. Curr. Opin. Chem. Biol. 2010, 14, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, D.; Lalot, T.; Brigodiot, M.; Maréchal, E. β-Diketones as key compounds in free-radical polymerization by enzyme-mediated initiation. Macromolecules 1998, 32, 70–72. [Google Scholar] [CrossRef]
- Kalra, B.; Gross, R.A. HRP-mediated polymerizations of acrylamide and sodium acrylate. Green Chem. 2002, 4, 174–178. [Google Scholar] [CrossRef]
- Xu, P.; Uyama, H.; Whitten, J.E.; Kobayashi, S.; Kaplan, D.L. Peroxidase-catalyzed in situ polymerization of surface orientated caffeic acid. J. Am. Chem. Soc. 2005, 127, 11745–11753. [Google Scholar] [CrossRef] [PubMed]
- Puskas, J.E.; Sen, M.Y.; Seo, K.S. Green polymer chemistry using nature's catalysts, enzymes. J. Polym. Sci. A 2009, 47, 2959–2976. [Google Scholar] [CrossRef]
- Emery, O.; Lalot, T.; Brigodiot, M.; Maréchal, E. Free-radical polymerization of acrylamide by horseradish peroxidase-mediated initiation. J. Polym. Sci. A 1997, 35, 3331–3333. [Google Scholar] [CrossRef]
- Ng, Y.-H.; di Lena, F.; Chai, C.L.L. Metalloenzymatic radical polymerization using alkyl halides as initiators. Polym. Chem. 2011, 2, 589–594. [Google Scholar] [CrossRef]
- Ng, Y.-H.; di Lena, F.; Chai, C.L.L. PolyPEGA with predetermined molecular weights from enzyme-mediated radical polymerization in water. Chem. Commun. 2011, 47, 6464–6466. [Google Scholar] [CrossRef] [PubMed]
- Silva, T.B.; Spulber, M.; Kocik, M.K.; Seidi, F.; Charan, H.; Rother, M.; Sigg, S.J.; Renggli, K.; Kali, G.; Bruns, N. Hemoglobin and red blood cells catalyze atom transfer radical polymerization. Biomacromolecules 2013, 14, 2703–2712. [Google Scholar] [CrossRef] [PubMed]
- Simakova, A.; Mackenzie, M.; Averick, S.E.; Park, S.; Matyjaszewski, K. Bioinspired iron-based catalyst for atom transfer radical polymerization. Angew. Chem. Int. Ed. 2013, 52, 12148–12151. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, K.; Yamamoto, K.; Kadokawa, J. Atom transfer radical polymerization of N-isopropylacrylamide by enzyme mimetic catalyst. Polymer 2013, 54, 1775–1778. [Google Scholar] [CrossRef]
- Gao, G.; Karaaslan, M.A.; Kadla, J.F.; Ko, F. Enzymatic synthesis of ionic responsive lignin nanofibres through surface poly(N-isopropylacrylamide) immobilization. Green Chem. 2014, 16, 3890–3898. [Google Scholar] [CrossRef]
- Zhou, H.; Jiang, W.; An, N.; Zhang, Q.; Xiang, S.; Wang, L.; Tang, J. Enzyme mimetic-catalyzed ATRP and its application in block copolymer synthesis combined with enzymatic ring-opening polymerization. RSC Adv. 2015, 5, 42728–42735. [Google Scholar] [CrossRef]
- Sigg, S.J.; Seidi, F.; Renggli, K.; Silva, T.B.; Kali, G.; Bruns, N. Horseradish peroxidase as a catalyst for atom transfer radical polymerization. Macromol. Rapid Commun. 2011, 32, 1710–1715. [Google Scholar] [CrossRef] [PubMed]
- Dinu, M.V.; Spulber, M.; Renggli, K.; Wu, D.; Monnier, C.A.; Petri-Fink, A.; Bruns, N. Filling polymersomes with polymers by peroxidase-catalyzed atom transfer radical polymerization. Macromol. Rapid Commun. 2015, 36, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Guan, S.; Li, P.; Luo, J.; Li, Y.; Huang, L.; Wang, G.; Zhu, L.; Fan, H.; Li, W.; Wang, L. A deuterohemin peptide extends lifespan and increases stress resistance in Caenorhabditis elegans. Free Radic. Res. 2010, 44, 813–820. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Li, Y.; Zhou, H.; Wang, L.; Cao, H.; Tang, J.; Li, W. PEGylation of deuterohaemin-alanine-histidine-threonine-valine-glutamic acid-lysine and its influence on activity, stability, and aggregation. J. Polym. Sci. Part. A 2013, 128, 706–711. [Google Scholar] [CrossRef]
- Moritz, M.; Geszke-Moritz, M. Mesoporous materials as multifunctional tools in biosciences: Principles and applications. Mat. Sci. Eng. C 2015, 49, 114–151. [Google Scholar] [CrossRef] [PubMed]
- Song, N.; Yang, Y.-W. Molecular and supramolecular switches on mesoporous silica nanoparticles. Chem. Soc. Rev. 2015, 44, 3474–3504. [Google Scholar] [CrossRef] [PubMed]
- Park, C.; Oh, K.; Lee, S.C.; Kim, C. Controlled release of guest molecules from mesoporous silica particles based on a ph-responsive polypseudorotaxane motif. Angew. Chem. Int. Ed. 2007, 46, 1455–1457. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Zhu, S.; Zhang, D. Grafting of thermo-responsive polymer inside mesoporous silica with large pore size using ATRP and investigation of its use in drug release. J. Mater. Chem. 2007, 17, 2428–2433. [Google Scholar] [CrossRef]
- Liu, R.; Zhao, X.; Wu, T.; Feng, P. Tunable redox-responsive hybrid nanogated ensembles. J. Am. Chem. Soc. 2008, 130, 14418–14419. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Shi, J. MSN Anti-cancer nanomedicines: Chemotherapy enhancement, overcoming of drug resistance, and metastasis inhibition. Adv. Mater. 2014, 26, 391–411. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Zhang, Y.S.; Pang, B.; Hyun, D.C.; Yang, M.; Xia, Y. Engineered nanoparticles for drug delivery in cancer therapy. Angew. Chem. Int. Ed. 2014, 53, 12320–12364. [Google Scholar] [CrossRef] [PubMed]
- Argyo, C.; Weiss, V.; Bräuchle, C.; Bein, T. Multifunctional mesoporous silica nanoparticles as a universal platform for drug delivery. Chem. Mater. 2014, 26, 435–451. [Google Scholar] [CrossRef]
- Aznar, E.; Oroval, M.; Pascual, L.; Murguía, J.R.; Martínez-Máñez, R.; Sancenón, F. Gated materials for on-command release of guest molecules. Chem. Rev. 2016, 116, 561–718. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.-L.; Yang, Y.-W.; Chen, D.-X.; Wang, G.; Zhou, Y.; Wang, C.-Y.; Stoddart, J.F. Mechanized silica nanoparticles based on pillar[5]arenes for on-command cargo release. Small 2013, 9, 3224–3229. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Sun, Y.-L.; Wang, L.; Ma, J.; Yang, Y.-W.; Gao, H. Nanoassembles constructed from mesoporous silica nanoparticles and surface-coated multilayer polyelectrolytes for controlled drug delivery. Microporous Mesoporous Mater. 2014, 185, 245–253. [Google Scholar] [CrossRef]
- Li, Q.-L.; Sun, Y.; Sun, Y.-L.; Wen, J.; Zhou, Y.; Bing, Q.-M.; Isaacs, L.D.; Jin, Y.; Gao, H.; Yang, Y.-W. Mesoporous silica nanoparticles coated by layer-by-layer self-assembly using cucurbit[7]uril for in vitro and in vivo anticancer drug release. Chem. Mater. 2014, 26, 6418–6431. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.-L.; Li, Q.-L.; Zhou, Y.; Jin, X.-Y.; Qi, A.-D.; Yang, Y.-W. Sugar and pH dual-responsive snap-top nanocarriers based on mesoporous silica-coated Fe3O4 magnetic nanoparticles for cargo delivery. Chem. Commun. 2015, 51, 4237–4240. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Wang, X.; Tang, J.; Yang, Y.-W. Tuning the growth, crosslinking, and gating effect of disulfide-containing PGMAs on the surfaces of mesoporous silica nanoparticles for redox/pH dual-controlled cargo release. Polym. Chem. 2016, 7, 2171–2179. [Google Scholar] [CrossRef]
- Yang, Y.-W.; Sun, Y.-L.; Song, N. Switchable Host–Guest Systems on Surfaces. Acc. Chem. Res. 2014, 47, 1950–1960. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xia, J.; Matyjaszewski, K. Controlled/“Living” radical polymerization of 2-(dimethylamino)ethyl methacrylate. Macromolecules 1998, 31, 5167–5169. [Google Scholar] [CrossRef] [PubMed]
- Zeng, F.; Shen, Y.; Zhu, S.; Pelton, R. Atom transfer radical polymerization of 2-(dimethylamino)ethyl methacrylate in aqueous media. J. Polym. Sci. Part. A 2000, 38, 3821–3827. [Google Scholar] [CrossRef]
- Yu, W.H.; Kang, E.T.; Neoh, K.G.; Zhu, S. Controlled grafting of well-defined polymers on hydrogen-terminated silicon substrates by surface-initiated atom transfer radical polymerization. J. Phys. Chem. B 2003, 107, 10198–10205. [Google Scholar] [CrossRef]
- Dong, H.; Matyjaszewski, K. ARGET ATRP of 2-(dimethylamino)ethyl methacrylate as an intrinsic reducing agent. Macromolecules 2008, 41, 6868–6870. [Google Scholar] [CrossRef]
- Zhao, Y.; Guo, K.; Wang, C.; Wang, L. Effect of inclusion complexation with cyclodextrin on the cloud point of poly(2-(dimethylamino)ethyl methacrylate) solution. Langmuir 2010, 26, 8966–8970. [Google Scholar] [CrossRef] [PubMed]
- Teoh, R.L.; Guice, K.B.; Loo, Y.-L. Atom transfer radical copolymerization of hydroxyethyl methacrylate and dimethylaminoethyl methacrylate in polar solvents. Macromolecules 2006, 39, 8609–8615. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, J.; Zhang, J.; Liu, J.; Yang, X.; Zhao, H. Exfoliated graphite oxide decorated by pdmaema chains and polymer particles. Langmuir 2009, 25, 11808–11814. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.-T.; Yu, Z.-Q.; Hong, C.-Y.; Pan, C.-Y. Biocompatible zwitterionic sulfobetaine copolymer-coated mesoporous silica nanoparticles for temperature-responsive drug release. Macromol. Rapid Commun. 2012, 33, 811–818. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Cheng, Q.; Jiang, Q.; Huang, Y.; Yang, Z.; Han, S.; Zhao, Y.; Guo, S.; Liang, Z.; Dong, A. Intracellular cleavable poly(2-dimethylaminoethyl methacrylate) functionalized mesoporous silica nanoparticles for efficient siRNA delivery in vitro and in vivo. Nanoscale 2013, 5, 4291–4301. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Tang, X.; Pei, M. Facile synthesis of PDMAEMA-coated hollow mesoporous silica nanoparticles and their pH-responsive controlled release. Microporous Mesoporous Mater. 2013, 173, 64–69. [Google Scholar] [CrossRef]
- Zou, H.; Yuan, W. Temperature- and redox-responsive magnetic complex micelles for controlled drug release. J. Mater. Chem. B 2015, 3, 260–269. [Google Scholar] [CrossRef]
- Li, Q.-L.; Xu, S.-H.; Zhou, H.; Wang, X.; Dong, B.; Gao, H.; Tang, J.; Yang, Y.-W. pH and Glutathione dual-responsive dynamic cross-linked supramolecular network on mesoporous silica nanoparticles for controlled anticancer drug release. ACS Appl. Mater. Interfaces 2015, 7, 28656–28664. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Zhu, Y.; Wang, Y. Dual-responsive drug delivery system with real time tunable release behavior. Microporous Mesoporous Mater. 2014, 200, 46–51. [Google Scholar] [CrossRef]
- Gao, Q.; Xu, Y.; Wu, D.; Sun, Y.; Li, X. pH-Responsive drug release from polymer-coated mesoporous silica spheres. J. Phys. Chem. C 2009, 113, 12753–12758. [Google Scholar] [CrossRef]
- Zhang, Y.; Ang, C.Y.; Li, M.; Tan, S.Y.; Qu, Q.; Luo, Z.; Zhao, Y. Polymer-coated hollow mesoporous silica nanoparticles for triple-responsive drug delivery. ACS Appl. Mater. Interfaces 2015, 7, 18179–18187. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.-T.; Hong, C.-Y.; Pan, C.-Y. Fabrication of PDEAEMA-coated mesoporous silica nanoparticles and pH-responsive controlled release. J. Phys. Chem. C 2010, 114, 12481–12486. [Google Scholar] [CrossRef]







| Materials | C (%) | H (%) | N (%) |
|---|---|---|---|
| MSN-OH | 4.67 | 2.07 | 0.42 |
| MSN-Br | 10.19 | 2.81 | 0.22 |
| MSN-PDMAEMA | 15.61 | 3.63 | 1.62 |
| Sample | BET surface area/m2·g−1 | Volume of pores/cm3·g−1 |
|---|---|---|
| MSN-OH | 829.32 | 0.98 |
| MSN-PDMAEMA | 337.22 | 0.69 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, H.; Wang, X.; Tang, J.; Yang, Y.-W. Surface Immobilization of pH-Responsive Polymer Brushes on Mesoporous Silica Nanoparticles by Enzyme Mimetic Catalytic ATRP for Controlled Cargo Release. Polymers 2016, 8, 277. https://doi.org/10.3390/polym8080277
Zhou H, Wang X, Tang J, Yang Y-W. Surface Immobilization of pH-Responsive Polymer Brushes on Mesoporous Silica Nanoparticles by Enzyme Mimetic Catalytic ATRP for Controlled Cargo Release. Polymers. 2016; 8(8):277. https://doi.org/10.3390/polym8080277
Chicago/Turabian StyleZhou, Hang, Xin Wang, Jun Tang, and Ying-Wei Yang. 2016. "Surface Immobilization of pH-Responsive Polymer Brushes on Mesoporous Silica Nanoparticles by Enzyme Mimetic Catalytic ATRP for Controlled Cargo Release" Polymers 8, no. 8: 277. https://doi.org/10.3390/polym8080277