Evolution of Network Structure and Mechanical Properties in Autonomous-Strengthening Dental Adhesive
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Formulations
2.3. Water Miscibility of Adhesive Formulations
2.4. Real-Time Conversion of C=C Bond
2.5. Preparation of Polymer Specimens
2.6. Water Sorption of Adhesive Polymer
2.7. DMA Test and Prony Series Evaluation
2.8. Leachable HEMA Study by High Performance Liquid Chromatography (HPLC)
2.9. Statistical Analysis
3. Results
4. Discussion
4.1. Role of Heating
4.2. Role of Water
4.3. Leaching Behavior of Aged Polymer Samples
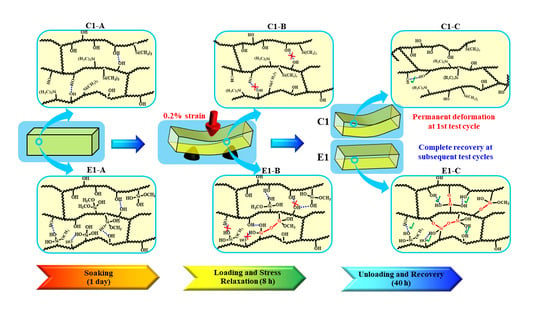
4.4. Network Structure Response to Cyclic Loading
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wen, J.Y.; Wilkes, G.L. Organic/inorganic hybrid network materials by the sol-gel approach. Chem. Mater. 1996, 8, 1667–1681. [Google Scholar] [CrossRef]
- Schottner, G. Hybrid sol-gel-derived polymers: Applications of multifunctional materials. Chem. Mater. 2001, 13, 3422–3435. [Google Scholar] [CrossRef]
- Kickelbick, G. Concepts for the incorporation of inorganic building blocks into organic polymers on a nanoscale. Prog. Polym. Sci. 2003, 28, 83–114. [Google Scholar] [CrossRef]
- Bosch, P.; DelMonte, F.; Mateo, J.L.; Levy, D. Photopolymerization of hydroxyethylmethacrylate in the formation of organic-inorganic hybrid sol-gel matrices. J. Polym. Sci. Polym. Chem. 1996, 34, 3289–3296. [Google Scholar] [CrossRef]
- Crivello, J.V.; Song, K.Y.; Choshal, R. Synthesis and photoinitiated cationic polymerization of organic-inorganic hybrid resins. Chem. Mater. 2001, 13, 1932–1942. [Google Scholar] [CrossRef]
- Dell’Erba, I.E.; Arenas, G.F.; Schroeder, W.F.; Asmussen, S.V.; Vallo, C.I. Hybrid organic-inorganic macromolecular photoinitiator system for visible-light photopolymerization. Prog. Org. Coat. 2014, 77, 1848–1853. [Google Scholar] [CrossRef]
- Kowalewska, A. Photoacid catalyzed sol-gel process. J. Mater. Chem. 2005, 15, 4997–5006. [Google Scholar] [CrossRef]
- Song, L.Y.; Ye, Q.; Ge, X.P.; Misra, A.; Tamerler, C.; Spencer, P. Self-strengthening hybrid dental adhesive via visible-light irradiation triple polymerization. RSC Adv. 2016, 6, 52434–52447. [Google Scholar] [CrossRef]
- Song, L.Y.; Ye, Q.; Ge, X.P.; Misra, A.; Spencer, P. Mimicking Nature: Self-strengthening Properties in a Dental Adhesive. Acta Biomater. 2016, 35, 138–152. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Shah, A.; Hsieh, A.J.; Haghighat, R.; Praveen, S.S.; Mukherjee, I.; Wei, E.; Zhang, Z.; Wei, Y. Characterization of poly(2-hydroxyethyl methacrylate-silica) hybrid materials with different silica contents. Polymer 2007, 48, 3982–3989. [Google Scholar] [CrossRef]
- Song, L.Y.; Ye, Q.; Ge, X.P.; Misra, A.; Tamerler, C.; Spencer, P. New silyl-functionalized BisGMA provides autonomous strengthening without leaching for dental adhesives. Acta Biomater. 2019, 83, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Song, L.Y.; Sarikaya, R.; Ye, Q.; Misra, A.; Tamerler, C.; Spencer, P. Multifunctional monomer acts as co-initiator and crosslinker to provide autonomous strengthening with enhanced hydrolytic stability in dental adhesives. Dent. Mater. 2020, 36, 284–295. [Google Scholar] [CrossRef] [PubMed]
- Delaviz, Y.; Finer, Y.; Santerre, J.P. Biodegradation of resin composites and adhesives by oral bacteria and saliva: A rationale for new material designs that consider the clinical environment and treatment challenges. Dent. Mater. 2014, 30, 16–32. [Google Scholar] [CrossRef] [PubMed]
- Scribante, A.; Gallo, S.; Turcato, B.; Trovati, F.; Gandini, P.; Sfondrini, M.F. Fear of the Relapse: Effect of Composite Type on Adhesion Efficacy of Upper and Lower Orthodontic Fixed Retainers: In Vitro Investigation and Randomized Clinical Trial. Polymers 2020, 12, 963. [Google Scholar] [CrossRef] [Green Version]
- Schwendicke, F.; Splieth, C.H.; Bottenberg, P.; Breschi, L.; Campus, G.; Domejean, S.; Ekstrand, K.; Giacaman, R.A.; Haak, R.; Hannig, M.; et al. How to intervene in the caries process in adults: Proximal and secondary caries? An EFCD-ORCA-DGZ expert Delphi consensus statement. Clin. Oral Investig. 2020, 24, 3315–3321. [Google Scholar] [CrossRef]
- Stewart, C.A.; Finer, Y. Biostable, antidegradative and antimicrobial restorative systems based on host-biomaterials and microbial interactions. Dent. Mater. 2019, 35, 36–52. [Google Scholar] [CrossRef]
- Spencer, P.; Ye, Q.; Song, L.Y.; Parthasarathy, R.; Boone, K.; Misra, A.; Tamerler, C. Threats to adhesive/dentin interfacial integrity and next generation bio-enabled multifunctional adhesives. J. Biomed. Mater. Res. Part B 2019, 107, 2673–2683. [Google Scholar] [CrossRef]
- Singh, V.; Misra, A.; Marangos, O.; Park, J.; Ye, Q.; Kieweg, S.L.; Spencer, P. Fatigue life prediction of dentin-adhesive interface using micromechanical stress analysis. Dent. Mater. 2011, 27, E187–E195. [Google Scholar] [CrossRef] [Green Version]
- Singh, V.; Misra, A.; Parthasarathy, R.; Ye, Q.; Park, J.; Spencer, P. Mechanical properties of methacrylate-based model dentin adhesives: Effect of loading rate and moisture exposure. J. Biomed. Mater. Res. Part B 2013, 101, 1437–1443. [Google Scholar] [CrossRef] [Green Version]
- Singh, V.; Misra, A.; Parthasarathy, R.; Ye, Q.; Spencer, P. Viscoelastic properties of collagen-adhesive composites under water-saturated and dry conditions. J. Biomed. Mater. Res. Part A 2015, 103, 646–657. [Google Scholar] [CrossRef]
- Parthasarathy, R.; Misra, A.; Song, L.Y.; Ye, Q.; Spencer, P. Structure-property relationships for wet dentin adhesive polymers. Biointerphases 2018, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Misra, A.; Parthasarathy, R.; Singh, V.; Spencer, P. Micro-poromechanics model of fluid-saturated chemically active fibrous media. Zamm-Z. Angew. Math. Mech. 2015, 95, 215–234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Misra, A.; Parthasarathy, R.; Ye, Q.; Singh, V.; Spencer, P. Swelling equilibrium of dentin adhesive polymers formed on the water-adhesive phase boundary: Experiments and micromechanical model. Acta Biomater. 2014, 10, 330–342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaudhuri, O.; Gu, L.; Klumpers, D.; Darnell, M.; Bencherif, S.A.; Weaver, J.C.; Huebsch, N.; Lee, H.P.; Lippens, E.; Duda, G.N.; et al. Hydrogels with tunable stress relaxation regulate stem cell fate and activity. Nat. Mater. 2016, 15, 326–334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vining, K.H.; Stafford, A.; Mooney, D.J. Sequential modes of crosslinking tune viscoelasticity of cell-instructive hydrogels. Biomaterials 2019, 188, 187–197. [Google Scholar] [CrossRef]
- Song, L.Y.; Ye, Q.; Ge, X.P.; Misra, A.; Laurence, J.S.; Berrie, C.L.; Spencer, P. Synthesis and evaluation of novel dental monomer with branched carboxyl acid group. J. Biomed. Mater. Res. Part B 2014, 102, 1473–1484. [Google Scholar] [CrossRef]
- Park, J.; Ye, Q.; Singh, V.; Kieweg, S.L.; Misra, A.; Spencer, P. Synthesis and evaluation of novel dental monomer with branched aromatic carboxylic acid group. J. Biomed. Mater. Res. Part B 2012, 100B, 569–576. [Google Scholar] [CrossRef] [Green Version]
- Parthasarathy, R.; Misra, A.; Park, J.; Ye, Q.; Spencer, P. Diffusion coefficients of water and leachables in methacrylate-based crosslinked polymers using absorption experiments. J. Mater. Sci. Mater. Med. 2012, 23, 1157–1172. [Google Scholar] [CrossRef] [Green Version]
- Ge, X.P.; Ye, Q.; Song, L.Y.; Laurence, J.S.; Misra, A.; Spencer, P. Probing the dual function of a novel tertiary amine compound in dentin adhesive formulations. Dent. Mater. 2016, 32, 519–528. [Google Scholar] [CrossRef] [Green Version]
- Park, S.W.; Schapery, R.A. Methods of interconversion between linear viscoelastic material functions. Part I—A numerical method based on Prony series. Int. J. Solids Struct. 1999, 36, 1653–1675. [Google Scholar] [CrossRef]
- Kwok, K.; Pellegrino, S. Folding, stowage, and deployment of viscoelastic tape springs. AIAA J. 2013, 51, 1908–1918. [Google Scholar] [CrossRef] [Green Version]
- Park, J.G.; Ye, Q.; Topp, E.M.; Lee, C.H.; Kostoryz, E.L.; Misra, A.; Spencer, P. Dynamic Mechanical Analysis and Esterase Degradation of Dentin Adhesives Containing a Branched Methacrylate. J. Biomed. Mater. Res. Part B 2009, 91B, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Ge, X.P.; Ye, Q.; Song, L.Y.; Misra, A.; Spencer, P. The influence of water on visible-light initiated free-radical/cationic ring-opening hybrid polymerization of methacrylate/epoxy: Polymerization kinetics, crosslinking structure and dynamic mechanical properties. RSC Adv. 2015, 5, 77791–77802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, L.Y.; Ye, Q.; Ge, X.P.; Spencer, P. Compositional design and optimization of dentin adhesive with neutralization capability. J. Dent. 2015, 43, 1132–1139. [Google Scholar] [CrossRef]
- Hashimoto, M. A Review-Micromorphological Evidence of Degradation in Resin-Dentin Bonds and Potential Preventional Solutions. J. Biomed. Mater. Res. Part B 2010, 92B, 268–280. [Google Scholar] [CrossRef] [PubMed]
- Tjaderhane, L.; Nascimento, F.D.; Breschi, L.; Mazzoni, A.; Tersariol, I.L.S.; Geraldeli, S.; Tezvergil-Mutluay, A.; Carrilho, M.; Carvalho, R.M.; Tay, F.R.; et al. Strategies to prevent hydrolytic degradation of the hybrid layer—A review. Dent. Mater. 2013, 29, 999–1011. [Google Scholar] [CrossRef] [Green Version]
- Frassetto, A.; Breschi, L.; Turco, G.; Marchesi, G.; Di Lenarda, R.; Tay, F.R.; Pashley, D.H.; Cadenaro, M. Mechanisms of degradation of the hybrid layer in adhesive dentistry and therapeutic agents to improve bond durability—A literature review. Dent. Mater. 2016, 32, E41–E53. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.H.; Song, S.; Chen, L.; Stafford, C.M.; Sun, J.R. Short-time dental resin biostability and kinetics of enzymatic degradation. Acta Biomater. 2018, 74, 326–333. [Google Scholar] [CrossRef]
- Amaral, F.L.B.; Colucci, V.; Palma-Dibb, R.G.; Corona, S.A.M. Assessment of in vitro methods used to promote adhesive interface degradation: A critical review. J. Esthet. Restor. Dent. 2007, 19, 340–353. [Google Scholar] [CrossRef]
- Chiaraputt, S.; Roongrujimek, P.; Sattabanasuk, V.; Panich, N.; Harnirattisai, C.; Senawongse, P. Biodegradation of all-in-one self-etch adhesive systems at the resin-dentin interface. Dent. Mater. J. 2011, 30, 814–826. [Google Scholar] [CrossRef] [Green Version]
- Spencer, P.; Ye, Q.; Park, J.; Topp, E.M.; Misra, A.; Marangos, O.; Wang, Y.; Bohaty, B.S.; Singh, V.; Sene, F.; et al. Adhesive/Dentin Interface: The Weak Link in the Composite Restoration. Ann. Biomed. Eng. 2010, 38, 1989–2003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yap, A.U.J.; Han, V.T.S.; Soh, M.S.; Siow, K.S. Elution of leachable components from composites after LED and halogen light irradiation. Oper. Dent. 2004, 29, 448–453. [Google Scholar]
- Hofmann, N.; Renner, J.; Hugo, B.; Klaiber, B. Elution of leachable components from resin composites after plasma arc vs. standard or soft-start halogen light irradiation. J. Dent. 2002, 30, 223–232. [Google Scholar] [CrossRef]
- Davidson, C.L.; Abdalla, A.I. Effect of thermal and mechanical load cycling on the marginal integrity of Class II resin composite restorations. Am. J. Dent. 1993, 6, 39–42. [Google Scholar] [PubMed]
- Ulker, M.; Ozcan, M.; Sengun, A.; Ozer, F.; Belli, S. Effect of Artificial Aging Regimens on the Performance of Self-Etching adhesives. J. Biomed. Mater. Res. Part B 2010, 93b, 175–184. [Google Scholar]
- Podgorski, M.; Matynia, T. Network structure/mechanical property relationship in multimethacrylates-derivatives of nadic anhydride. J. Appl. Polym. Sci. 2008, 109, 2624–2635. [Google Scholar] [CrossRef]
- Song, L.Y.; Ye, Q.; Ge, X.P.; Misra, A.; Tamerler, C.; Spencer, P. Fabrication of hybrid crosslinked network with buffering capabilities and autonomous strengthening characteristics for dental adhesives. Acta Biomater. 2018, 67, 111–121. [Google Scholar] [CrossRef] [Green Version]
- Kaleema, M.; Masouras, K.; Satterthwaite, J.D.; Silikas, N.; Watts, D.C. Viscoelastic stability of resin-composites under static and dynamic loading. Dent. Mater. 2012, 28, E15–E18. [Google Scholar] [CrossRef]
- Pavlinec, J.; Moszner, N. Dark reactions of free radicals crosslinked polymer networks trapped in densely after photopolymerization. J. Appl. Polym. Sci. 2003, 89, 579–588. [Google Scholar] [CrossRef]
- Morita, S. Hydrogen-bonds structure in poly(2-hydroxyethyl methacrylate) studied by temperature-dependent infrared spectroscopy. Front. Chem. 2014, 2, 10. [Google Scholar] [CrossRef] [Green Version]
- Xie, Y.J.; Hill, C.A.S.; Xiao, Z.F.; Militz, H.; Mai, C. Silane coupling agents used for natural fiber/polymer composites: A review. Compos. Part A Appl. Sci. 2010, 41, 806–819. [Google Scholar] [CrossRef]
- Wei, Y.; Jin, D.L.; Xu, J.G.; Baran, G.; Qiu, K.Y. Mechanical properties of interface-free polyacrylate-silica hybrid sol-gel materials for potential dental applications. Polym. Adv. Technol. 2001, 12, 361–368. [Google Scholar] [CrossRef]
- Munstedt, H. Rheological properties and molecular structure of polymer melts. Soft Matter 2011, 7, 2273–2283. [Google Scholar] [CrossRef]
- Ben Ammar, N.E.; Saied, T.; Barbouche, M.; Hosni, F.; Hamzaoui, A.H.; Sen, M. A comparative study between three different methods of hydrogel network characterization: Effect of composition on the crosslinking properties using sol-gel, rheological and mechanical analyses. Polym. Bull. 2018, 75, 3825–3841. [Google Scholar] [CrossRef]
- Wang, S.B.; Tang, H.B.; Guo, J.C.; Wang, K.J. Effect of pH on the rheological properties of borate crosslinked hydroxypropyl guar gum hydrogel and hydroxypropyl guar gum. Carbohydr. Polym. 2016, 147, 455–463. [Google Scholar] [CrossRef]








| Component (wt%) | C1 | E1 |
|---|---|---|
| HEMA | 58 | 58 |
| BisGMA | 30 | 30 |
| MES | 10 | - |
| MPS | - | 10 |
| CQ | 0.5 | 0.5 |
| EDMAB | 0.5 | 0.5 |
| DPIHP | 1.0 | 1.0 |
| Type | Sample | No. | Rubbery Modulus (MPa) | Tg (°C) | ζ (× 10–5 Pa–1 K) |
|---|---|---|---|---|---|
| Non-treated | C1 | 1st | 11.9A ± 0.5 | 111.8A ± 0.6 | 3.26A ± 0.12 |
| 2nd | 12.8A ± 0.3 | 112.3A ± 0.8 | 3.07A ± 0.15 | ||
| E1 | 1st | 13.1A ± 0.6 | 111.2A ± 0.5 | 2.97A ± 0.12 | |
| 2nd | 26.7B ± 1.2 | 118.9B ± 0.3 | 1.46B ± 0.05 | ||
| Treated | C1 | 1 day | 11.8A ± 0.5 | 120.2B ± 0.3 | 3.38A ± 0.20 |
| 3 days | 12.0A ± 0.6 | 122.2B ± 0.7 | 3.29A ± 0.18 | ||
| 5 days | 11.1A ± 0.9 | 123.2B ± 0.1 | 3.59A ± 0.29 | ||
| E1 | 1 day | 37.4B ± 3.3 | 130.4B ± 1.9 | 1.08B ± 0.09 | |
| 3 days | 42.6B ± 0.7 | 133.9B ± 0.8 | 0.96B ± 0.01 | ||
| 5 days | 39.5B ± 0.4 | 133.1B ± 0.5 | 1.03B ± 0.01 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarikaya, R.; Song, L.; Ye, Q.; Misra, A.; Tamerler, C.; Spencer, P. Evolution of Network Structure and Mechanical Properties in Autonomous-Strengthening Dental Adhesive. Polymers 2020, 12, 2076. https://doi.org/10.3390/polym12092076
Sarikaya R, Song L, Ye Q, Misra A, Tamerler C, Spencer P. Evolution of Network Structure and Mechanical Properties in Autonomous-Strengthening Dental Adhesive. Polymers. 2020; 12(9):2076. https://doi.org/10.3390/polym12092076
Chicago/Turabian StyleSarikaya, Rizacan, Linyong Song, Qiang Ye, Anil Misra, Candan Tamerler, and Paulette Spencer. 2020. "Evolution of Network Structure and Mechanical Properties in Autonomous-Strengthening Dental Adhesive" Polymers 12, no. 9: 2076. https://doi.org/10.3390/polym12092076