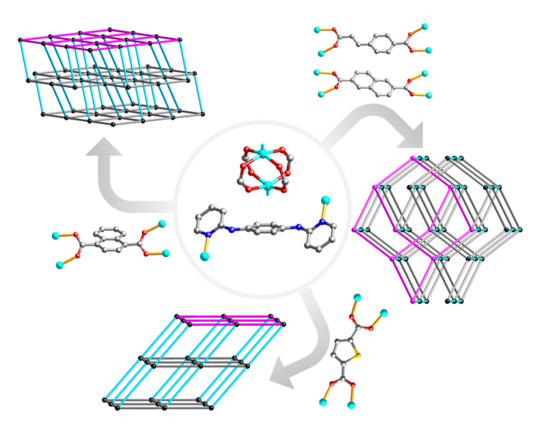
Paddlewheel SBU based Zn MOFs: Syntheses, Structural Diversity, and CO2 Adsorption Properties
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials and Instruments
2.2. Synthesis of {[Zn2(2,6-ndc)2(2-Pn)]·DMF}n (1)
2.3. Synthesis of {[Zn2(cca)2(2-Pn)]·DMF}n (2)
2.4. Synthesis of {[Zn2(thdc)2(2-Pn)]·3DMF}n (3)
2.5. Synthesis of {[Zn2(1,4-ndc)2(2-Pn)]·1.5DMF}n (4)
2.6. X-Ray Data Collection and Structure Refinement
3. Results and Discussion
3.1. Crystal Structures of {[Zn2(2,6-ndc)2(2-Pn)]·DMF}n (1) and {[Zn2(cca)2(2-Pn)]·DMF}n (2)
3.2. Crystal Structure of {[Zn2(thdc)2(2-Pn)]·3DMF}n (3)
3.3. Crystal Structure of {[Zn2(1,4-ndc)2(2-Pn)]·1.5DMF}n (4)
3.4. Powder X-Ray Diffraction and Thermogravimetric Analysis
3.5. CO2 Adsorption Properties
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Luo, X.-L.; Yin, Z.; Zeng, M.-H.; Kurmoo, M. The construction, structures, and functions of pillared layer metal–organic frameworks. Inorg. Chem. Front. 2016, 3, 1208–1226. [Google Scholar] [CrossRef]
- Trickett, C.A.; Helal, A.; Al-Maythalony, B.A.; Yamani, Z.H.; Cordova, K.E.; Yaghi, O.M. The chemistry of metal–organic frameworks for CO2 capture, regeneration and conversion. Nat. Rev. Mater. 2017, 2, 17045. [Google Scholar] [CrossRef]
- Yu, J.; Xie, L.-H.; Li, J.-R.; Ma, Y.; Seminario, J.M.; Balbuena, P.B. CO2 Capture and Separations Using MOFs: Computational and Experimental Studies. Chem. Rev. 2017, 117, 9674–9754. [Google Scholar] [CrossRef] [PubMed]
- Queen, W.L.; Hudson, M.R.; Bloch, E.D.; Mason, J.A.; Gonzalez, M.I.; Lee, J.S.; Gygi, D.; Howe, J.D.; Lee, K.; Darwish, T.A.; et al. Comprehensive study of carbon dioxide adsorption in the metal–organic frameworks M2(dobdc) (M = Mg, Mn, Fe, Co, Ni, Cu, Zn). Chem. Sci. 2014, 5, 4569–4581. [Google Scholar] [CrossRef]
- Gosselin, E.J.; Lorzing, G.R.; Trump, B.A.; Brown, C.M.; Bloch, E.D. Gas adsorption in an isostructural series of pillared coordination cages. Chem. Commun. 2018, 54, 6392–6395. [Google Scholar] [CrossRef] [PubMed]
- Noori, Y.; Akhbari, K. Post-synthetic ion-exchange process in nanoporous metal–organic frameworks; an effective way for modulating their structures and properties. RSC Adv. 2017, 7, 1782–1808. [Google Scholar] [CrossRef] [Green Version]
- Brozek, C.K.; Bellarosa, L.; Soejima, T.; Clark, T.V.; López, N.; Dincă, M. Solvent-Dependent Cation Exchange in Metal–Organic Frameworks. Chem. Eur. J. 2014, 20, 6871–6874. [Google Scholar] [CrossRef]
- Lustig, W.P.; Mukherjee, S.; Rudd, N.D.; Desai, A.V.; Li, J.; Ghosh, S.K. Metal–organic frameworks: Functional luminescent and photonic materials for sensing applications. Chem. Soc. Rev. 2017, 46, 3242–3285. [Google Scholar] [CrossRef]
- Hu, Z.; Deibert, B.J.; Li, J. Luminescent metal–organic frameworks for chemical sensing and explosive detection. Chem. Soc. Rev. 2014, 43, 5815–5840. [Google Scholar] [CrossRef]
- Zhu, L.; Liu, X.-Q.; Jiang, H.-L.; Sun, L.-B. Metal–Organic Frameworks for Heterogeneous Basic Catalysis. Chem. Rev. 2017, 117, 8129–8176. [Google Scholar] [CrossRef]
- Espallargas, G.M.; Coronado, E. Magnetic functionalities in MOFs: From the framework to the pore. Chem. Soc. Rev. 2018, 47, 533–557. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zheng, M.; Xie, Z. Nanoscale metal–organic frameworks for drug delivery: A conventional platform with new promise. J. Mater. Chem. B 2018, 6, 707–717. [Google Scholar] [CrossRef]
- Tranchemontagne, D.J.; Mendoza-Cortés, J.L.; O’Keeffea, M.; Yaghi, O.M. Secondary building units, nets and bonding in the chemistry of metal–organic frameworks. Chem. Soc. Rev. 2009, 38, 1257–1283. [Google Scholar] [CrossRef] [PubMed]
- Carson, C.G.; Hardcastle, K.; Schwartz, J.; Liu, X.; Hoffmann, C.; Gerhardt, R.A.; Tannenbaum, R. Synthesis and Structure Characterization of Copper Terephthalate Metal–Organic Frameworks. Eur. J. Inorg. Chem. 2009, 2338–2343. [Google Scholar] [CrossRef]
- Mori, W.; Sato, T.; Kato, C.N.; Takei, T.; Ohmura, T. Discovery and development of microporous metal carboxylates. Chem. Rec. 2005, 5, 336–351. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Juricek, M.; Coudert, F.X.; Vermeulen, N.A.; Grunder, S.; Dailly, A.; Lewis, W.; Blake, A.J.; Stoddart, J.F.; Schroder, M. Non-Interpenetrated Metal–Organic Frameworks Based on Copper(II) Paddlewheel and Oligoparaxylene-Isophthalate Linkers: Synthesis, Structure, and Gas Adsorption. J. Am. Chem. Soc. 2016, 138, 3371–3381. [Google Scholar] [CrossRef] [PubMed]
- Luo, F.; Yan, C.; Dang, L.; Krishna, R.; Zhou, W.; Wu, H.; Dong, X.; Han, Y.; Hu, T.L.; O’Keeffe, M.; et al. UTSA-74: A MOF-74 Isomer with Two Accessible Binding Sites per Metal Center for Highly Selective Gas Separation. J. Am. Chem. Soc. 2016, 138, 5678–5684. [Google Scholar] [CrossRef]
- Dhankhar, S.S.; Kaur, M.; Nagaraja, C.M. Green Synthesis of a Microporous, Partially Fluorinated ZnII Paddlewheel Metal–Organic Framework: H2/CO2 Adsorption Behavior and Solid-State Conversion to a ZnO–C Nanocomposite. Eur. J. Inorg. Chem. 2015, 5669–5676. [Google Scholar] [CrossRef]
- Li, P.-Z.; Wang, X.-J.; Liu, J.; Liang, J.; Chen, J.Y.J.; Zhao, Y. Two metal–organic frameworks sharing the same basic framework show distinct interpenetration degrees and different performances in CO2 catalytic conversion. CrystEngComm 2017, 19, 4157–4161. [Google Scholar] [CrossRef]
- Chang, C.-C.; Huang, Y.-C.; Huang, S.-M.; Wu, J.-Y.; Liu, Y.-H.; Lu, K.-L. Pre-Synthesized and In-situ Generated Tetrazolate Ligand in the Design of Chiral Cadmium Coordination Polymer. Cryst. Growth Des. 2012, 12, 3825–3828. [Google Scholar] [CrossRef]
- Wu, J.-Y.; Ding, M.-T.; Wen, Y.-S.; Liu, Y.-H.; Lu, K.-L. Alkali Cation (K+, Cs+)-Induced Dissolution/Reorganization of Porous Metal–Carboxylate Coordination Networks in Water. Chem. Eur. J. 2009, 15, 3604–3614. [Google Scholar] [CrossRef] [PubMed]
- Kondo, M.; Takashima, Y.; Seo, J.; Kitagawa, S.; Furukawa, S. Control over the nucleation process determines the framework topology of porous coordination polymers. CrystEngComm 2010, 12, 2350–2353. [Google Scholar] [CrossRef]
- Zhao, D.; Liu, X.-H.; Guo, J.-H.; Xu, H.-J.; Zhao, Y.; Lu, Y.; Sun, W.-Y. Porous Metal–Organic Frameworks with Chelating Multiamine Sites for Selective Adsorption and Chemical Conversion of Carbon Dioxide. Inorg. Chem. 2018, 57, 2695–2704. [Google Scholar] [CrossRef] [PubMed]
- Kochetygov, I.; Bulut, S.; Asgari, M.; Queen, W.L. Selective CO2 adsorption by a new metal–organic framework: Synergy between open metal sites and a charged imidazolinium backbone. Dalton Trans. 2018, 47, 10527–10535. [Google Scholar] [CrossRef]
- Wu, Y.-L.; Qian, J.; Yang, G.-P.; Yang, F.; Liang, Y.-T.; Zhang, W.-Y.; Wang, Y.-Y. High CO2 Uptake Capacity and Selectivity in a Fascinating Nanotube-Based Metal–Organic Framework. Inorg. Chem. 2017, 56, 908–913. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Lu, Z.; Zheng, K.; Wang, J.; Zheng, X.; Pan, Y.; You, X.; Bai, J. Fine-Tuning Pore Size by Shifting Coordination Sites of Ligands and Surface Polarization of Metal–Organic Frameworks to Sharply Enhance the Selectivity for CO2. J. Am. Chem. Soc. 2013, 135, 562–565. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-H.; Huang, H.-Y.; Liu, Y.-H.; Luo, T.-T.; Lee, G.-H.; Peng, S.-M.; Jiang, J.-C.; Chao, I.; Lu, K.-L. Cooperative Effect of Unsheltered Amide Groups on CO2 Adsorption Inside Open-Ended Channels of a Zinc(II)–Organic Framework. Inorg. Chem. 2013, 52, 3962–3968. [Google Scholar] [CrossRef]
- Lee, C.-H.; Wu, J.-Y.; Lee, G.-H.; Peng, S.-M.; Jiang, J.-C.; Lu, K.-L. Amide-containing zinc(II) metal–organic layered networks: A structure–CO2 capture relationship. Inorg. Chem. Front. 2015, 2, 477–484. [Google Scholar] [CrossRef]
- Lee, C.-H.; Huang, H.-Y.; Lee, J.-J.; Huang, C.-Y.; Kao, Y.-C.; Lee, G.-H.; Peng, S.-M.; Jiang, J.-C.; Chao, I.; Lu, K.-L. Amide–CO2 Interaction Induced Gate-Opening Behavior for CO2 Adsorption in 2-Fold Interpenetrating Framework. ChemistrySelect 2016, 1, 2923–2929. [Google Scholar] [CrossRef]
- Alduhaish, O.; Wang, H.; Li, B.; Arman, H.D.; Nesterov, V.; Alfooty, K.; Chen, B. A Threefold Interpenetrated Pillared-Layer Metal–Organic Framework for Selective Separation of C2H2/CH4 and CO2/CH4. ChemPlusChem 2016, 81, 764–769. [Google Scholar] [CrossRef]
- Tseng, T.-W.; Lee, L.-W.; Luo, T.-T.; Chien, P.-H.; Liu, Y.-H.; Lee, S.-L.; Wang, C.-M.; Lu, K.-L. Gate-Opening upon CO2 Adsorption on a Metal–Organic Framework That Mimics a Natural Stimuli-Response System. Dalton Trans. 2017, 46, 14728–14732. [Google Scholar] [CrossRef] [PubMed]
- Bensemann, I.; Gdaniec, M.; Połoński, T. Supramolecular structures formed by 2-aminopyridine derivatives. Part I. Hydrogen-bonding networks via N–H⋯N interactions and the conformational polymorphism of N,N’-bis(2-pyridyl)aryldiamines. New J. Chem. 2002, 26, 448–456. [Google Scholar] [CrossRef]
- Wicher, B.; Gdaniec, M. A third polymorph of N,N’-bis-(pyridin-2-yl)benzene-1,4-diamine. Acta Crystallogr. Sect. E 2011, 67, o3095. [Google Scholar] [CrossRef]
- Krause, L.; Herbst-Irmer, R.; Sheldrick, G.M.; Stalke, D. Comparison of silver and molybdenum microfocus X-ray sources for single-crystal structure determination. J. Appl. Crystallogr. 2015, 48, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. Sect. A 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Farrugia, L.J. WinGX and ORTEP for Windows: An update. J. Appl. Crystallogr. 2012, 45, 849–854. [Google Scholar] [CrossRef]
- Spek, A.L. PLATON SQUEEZE: A tool for the calculation of the disordered solvent contribution to the calculated structure factors. Acta Crystallogr. Sect. C 2015, 71, 9–18. [Google Scholar] [CrossRef]
- Spek, A.L. Structure validation in chemical crystallography. Acta Crystallogr. Sect. D 2009, 65, 148–155. [Google Scholar] [CrossRef] [Green Version]
- Sen, S.; Neogi, S.; Aijaz, A.; Xu, Q.; Bharadwaj, P.K. Construction of Non-Interpenetrated Charged Metal–Organic Frameworks with Doubly Pillared Layers: Pore Modification and Selective Gas Adsorption. Inorg. Chem. 2014, 53, 7591–7598. [Google Scholar] [CrossRef]
- Bureekaew, S.; Sato, H.; Matsuda, R.; Kubota, Y.; Hirose, R.; Kim, J.; Kato, K.; Takata, M.; Kitagawa, S. Control of Interpenetration for Tuning Structural Flexibility Influences Sorption Properties. Angew. Chem. Int. Ed. 2010, 49, 7660–7664. [Google Scholar] [CrossRef]
- Schneemann, A.; Vervoorts, P.; Hante, I.; Tu, M.; Wannapaiboon, S.; Sternemann, C.; Paulus, M.; Wieland, D.C.F.; Henke, S.; Fischer, R.A. Different Breathing Mechanisms in Flexible Pillared-Layered Metal–Organic Frameworks: Impact of the Metal Center. Chem. Mater. 2018, 30, 1667–1676. [Google Scholar] [CrossRef]
- Chen, D.-M.; Zhang, X.-P.; Shi, W.; Cheng, P. Tuning Two-Dimensional Layer to Three-Dimensional Pillar-Layered Metal–Organic Frameworks: Polycatenation and Interpenetration Behaviors. Cryst. Growth Des. 2014, 14, 6261–6268. [Google Scholar] [CrossRef]
- Hu, X.-L.; Liu, F.-H.; Wang, H.-N.; Qin, C.; Sun, C.-Y.; Su, Z.-M.; Liu, F.-C. Controllable synthesis of isoreticular pillared-layer MOFs: Gas adsorption, iodine sorption and sensing small molecules. J. Mater. Chem. A 2014, 2, 14827–14834. [Google Scholar] [CrossRef]
- Hu, X.-L.; Gong, Q.-H.; Zhong, R.-L.; Wang, X.-L.; Qin, C.; Wang, H.; Li, J.; Shao, K.-Z.; Su, Z.-M. Evidence of Amine–CO2 Interactions in Two Pillared-Layer MOFs Probed by X-ray Crystallography. Chem. Eur. J. 2015, 21, 7238–7244. [Google Scholar] [CrossRef] [PubMed]
- Demessence, A.; D’Alessandro, D.M.; Foo, M.L.; Long, J.R. Strong CO2 Binding in a Water-Stable, Triazolate-Bridged Metal–Organic Framework Functionalized with Ethylenediamine. J. Am. Chem. Soc. 2009, 131, 8784–8786. [Google Scholar] [CrossRef] [PubMed]








| Compounds | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| Empirical Formula | C40H26N4O8Zn2 | C36H26N4O8Zn2 | C34H32N6O10S2Zn2 | C44.5H36.5N5.5O9.5Zn2 |
| Mw | 821.39 | 773.35 | 879.51 | 931.03 |
| Crystal system | Monoclinic | Monoclinic | Monoclinic | Triclinic |
| Space group | C2/c | C2/c | C2/c | P |
| a/Å | 8.2847(17) | 8.0307(16) | 19.710(4) | 10.697(2) |
| b/Å | 34.127(7) | 34.118(7) | 15.618(3) | 14.488(3) |
| c/Å | 13.592(3) | 13.527(3) | 13.591(3) | 14.912(3) |
| α/° | 90 | 90 | 90 | 81.38(3) |
| β/° | 93.66(3) | 96.50(3) | 106.47(3) | 79.39(3) |
| γ/° | 90 | 90 | 90 | 76.04(3) |
| V/Å3 | 3835.2(13) | 3682.5(14) | 4011.9(15) | 2191.0(8) |
| Z | 4 | 4 | 4 | 2 |
| T/K | 150(2) | 150(2) | 150(2) | 150(2) |
| λ/Å | 0.71073 | 0.71073 | 0.71073 | 0.71073 |
| Dcalc/g cm−3 | 1.423 | 1.395 | 1.456 | 1.411 |
| F000 | 1672 | 1576 | 1800 | 956 |
| μ/mm−1 | 1.307 | 1.357 | 1.360 | 1.157 |
| R1a, wR2b (I > 2σ (I)) | 0.0377, 0.1032 | 0.0426, 0.1208 | 0.0313, 0.0862 | 0.0574, 0.1674 |
| R1a, wR2b (all data) | 0.0563, 0.1229 | 0.0480, 0.1253 | 0.0413, 0.1009 | 0.0915, 0.1909 |
| Zn MOFs | CO2 Adsorption Amount (cm3/g) | Reference |
|---|---|---|
| [Zn2(2,6-ndc)2(2-Pn)] (1′) | 55.1 | this work |
| [Zn2(cca)2(2-Pn)] (2′) | 84.6 | this work |
| [Zn2(1,4-ndc)2(2-Pn)] (4′) | 64.3 | this work |
| [Zn2(L)(bpy)2](NO3) | 50.6 | [40] |
| [Zn2(L)(bpe)2](NO3) | 74.9 | [40] |
| [Zn2(L)(bpb)2](NO3) | 146.9 | [40] |
| [Zn2(btdc)(bpy)2] (3-fold interpenetration) | 55 | [41] |
| [Zn2(btdc)(bpy)2] (2-fold interpenetration) | 201 | [41] |
| [Zn2(bme-1,4-bdc)(dabco)2] | 80.2 | [42] |
| [Zn2(bpydb)(bpy)] | 83 | [43] |
| [Zn2(fma)(trz)2] | 188.5 | [44] |
| [Zn2(1,4-bdc)(trz)2] | 119.4 | [45] |
| [Zn2(NH2-1,4-bdc)(trz)2] | 136.3 | [44,45] |
| [Zn2(Br-1,4-bdc)(trz)2] | 108.9 | [44] |
| [Zn2(1,4-ndc)(trz)2] | 86.88 | [44] |
| [Zn2(oba)(trz)2] | 70.68 | [44] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, T.-R.; Lee, C.-H.; Lan, Y.-C.; Mendiratta, S.; Lai, L.-L.; Wu, J.-Y.; Chi, K.-M.; Lu, K.-L. Paddlewheel SBU based Zn MOFs: Syntheses, Structural Diversity, and CO2 Adsorption Properties. Polymers 2018, 10, 1398. https://doi.org/10.3390/polym10121398
Lin T-R, Lee C-H, Lan Y-C, Mendiratta S, Lai L-L, Wu J-Y, Chi K-M, Lu K-L. Paddlewheel SBU based Zn MOFs: Syntheses, Structural Diversity, and CO2 Adsorption Properties. Polymers. 2018; 10(12):1398. https://doi.org/10.3390/polym10121398
Chicago/Turabian StyleLin, Ting-Ru, Cheng-Hua Lee, Yi-Chen Lan, Shruti Mendiratta, Long-Li Lai, Jing-Yun Wu, Kai-Ming Chi, and Kuang-Lieh Lu. 2018. "Paddlewheel SBU based Zn MOFs: Syntheses, Structural Diversity, and CO2 Adsorption Properties" Polymers 10, no. 12: 1398. https://doi.org/10.3390/polym10121398