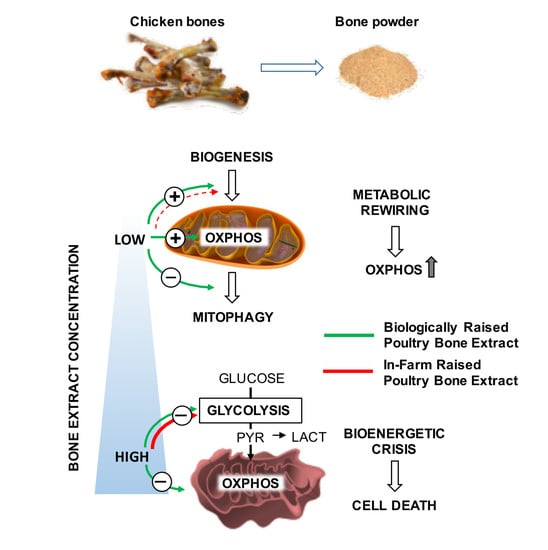
Effect of Chicken Bone Extracts on Metabolic and Mitochondrial Functions of K562 Cell Line
Abstract
:1. Introduction
2. Results
2.1. Effect of Chicken Bone Powder Extracts and Oxytetracycline on K562 Cell Viability
2.2. Effect of Chicken Bone Powder Extracts and Oxytetracycline on Mitochondrial Respiration and Glycolysis in K562 Cell
2.3. Effect of Chicken Bone Powder Extracts and Oxytetracycline on the Mitochondrial Membrane Potential and on Peroxide Production in K562 Cell
2.4. Effect of Chicken Bone Powder Extracts and Oxytetracycline on the Expression Level of Factors Involved in the Mitochondrial Turn-Over
3. Materials and Methods
3.1. Chicken Bone Extract
3.2. Cell Culture and Compound Treatment
3.3. Metabolic Flux Analysis
3.4. Confocal Microscopy Imaging
3.5. RNA Isolation and Quantitative RT-PCR
3.6. Statistical Analysis
4. Discussion
5. Study Limitations
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Di Cerbo, A.; Pezzuto, F.; Scarano, A.; Guidetti, G.; Canello, S. The contradictory world of tetracyclines. Panminerva Med. 2018. [Google Scholar] [CrossRef]
- Di Cerbo, A.; Pezzuto, F.; Guidetti, G.; Canello, S.; Corsi, L. Tetracyclines: Insights and Updates of their Use in Human and Animal Pathology and their Potential Toxicity. Open Biochem. J. 2018, 13, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Palmieri, B.; Di Cerbo, A.; Laurino, C. Antibiotic treatments in zootechnology and effects induced on the food chain of domestic species and, comparatively, the human specie. Nutr. Hosp. 2014, 29, 1427–1433. [Google Scholar] [CrossRef] [PubMed]
- Chukwudi, C.U. rRNA Binding Sites and the Molecular Mechanism of Action of the Tetracyclines. Antimicrob. Agents Chemother. 2016, 60, 4433–4441. [Google Scholar] [CrossRef] [Green Version]
- Batabyal, B. Urinary Tract Infection in Children and Antimicrobial Resistance Pattern: Children UTI and Antibiotic Resistance; Educreation Publishing: Chhattisgarh, India, 2019; p. 197. [Google Scholar]
- Chatzispyrou, I.A.; Held, N.M.; Mouchiroud, L.; Auwerx, J.; Houtkooper, R.H. Tetracycline antibiotics impair mitochondrial function and its experimental use confounds research. Cancer Res. 2015, 75, 4446–4449. [Google Scholar] [CrossRef] [Green Version]
- Wallace, D.C. Why do we still have a maternally inherited mitochondrial DNA? Insights from evolutionary medicine. Annu. Rev. Biochem. 2007, 76, 781–821. [Google Scholar] [CrossRef] [Green Version]
- Giachin, G.; Bouverot, R.; Acajjaoui, S.; Pantalone, S.; Soler-Lopez, M. Dynamics of Human Mitochondrial Complex I Assembly: Implications for Neurodegenerative Diseases. Front Mol. Biosci. 2016, 3, 43. [Google Scholar] [CrossRef] [Green Version]
- Official Journal of the European Union. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/HTML/?uri=CELEX:32010R0037&from=EN,%20Ed.%202009 (accessed on 1 June 2020).
- Gazzetta Ufficiale. Available online: https://www.camera.it/parlam/leggi/deleghe/testi/06193dl.htm (accessed on 1 June 2020).
- EFSA. Scientific Opinion on the public health risks related to mechanically separated meat (MSM) derived from poultry and swine. Efsa J. 2013, 11, 3137. [Google Scholar] [CrossRef]
- Di Cerbo, A.; Canello, S.; Guidetti, G.; Fiore, F.; Corsi, L.; Rubattu, N.; Testa, C.; Cocco, R. Adverse food reactions in dogs due to antibiotic residues in pet food: A preliminary study. Vet. Ital. 2018, 54, 137–146. [Google Scholar] [CrossRef]
- Mazzeranghi, F.; Zanotti, C.; Di Cerbo, A.; Verstegen, J.P.; Cocco, R.; Guidetti, G.; Canello, S. Clinical efficacy of nutraceutical diet for cats with clinical signs of cutaneus adverse food reaction (CAFR). Pol. J. Vet. Sci. 2017, 20, 269–276. [Google Scholar] [CrossRef] [Green Version]
- Di Cerbo, A.; Scarano, A.; Pezzuto, F.; Guidetti, G.; Canello, S.; Pinetti, D.; Genovese, F.; Corsi, L. Oxytetracycline-Protein Complex: The Dark Side of Pet Food. Open Public Health J. 2018, 11, 162–169. [Google Scholar] [CrossRef]
- Official Journal of the European Union. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/HTML/?uri=CELEX:32005R2074&from=IT,%20Ed.%202005 (accessed on 1 June 2020).
- Di Cerbo, A.; Canello, S.; Guidetti, G.; Laurino, C.; Palmieri, B. Unusual antibiotic presence in gym trained subjects with food intolerance; a case report. Nutr. Hosp. 2014, 30, 395–398. [Google Scholar] [CrossRef] [PubMed]
- Graham, F.; Paradis, L.; Begin, P.; Paradis, J.; Babin, Y.; Des Roches, A. Risk of allergic reaction and sensitization to antibiotics in foods. Ann. Allergy Asthma Immunol. 2014, 113, 329–330. [Google Scholar] [CrossRef] [PubMed]
- Di Cerbo, A.; Guidetti, G.; Canello, S.; Cocco, R.A. possible correlation between diet, serum oxytetracycline concentration, and onset of reproductive disturbances in bitches: Clinical observations and preliminary results. Turk. J. Vet. Anim. Sci. 2019, 43, 523–531. [Google Scholar] [CrossRef]
- Di Cerbo, A.; Palatucci, A.T.; Rubino, V.; Centenaro, S.; Giovazzino, A.; Fraccaroli, E.; Cortese, L.; Ruggiero, G.; Guidetti, G.; Canello, S.; et al. Toxicological Implications and Inflammatory Response in Human Lymphocytes Challenged with Oxytetracycline. J. Biochem. Mol. Toxicol. 2016, 30, 170–177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Odore, R.; De Marco, M.; Gasco, L.; Rotolo, L.; Meucci, V.; Palatucci, A.T.; Rubino, V.; Ruggiero, G.; Canello, S.; Guidetti, G.; et al. Cytotoxic effects of oxytetracycline residues in the bones of broiler chickens following therapeutic oral administration of a water formulation. Poult. Sci. 2015, 94, 1979–1985. [Google Scholar] [CrossRef]
- Di Cerbo, A.; Rubino, V.; Morelli, F.; Ruggiero, G.; Landi, R.; Guidetti, G.; Canello, S.; Terrazzano, G.; Alessandrini, A. Mechanical phenotyping of K562 cells by the Micropipette Aspiration Technique allows identifying mechanical changes induced by drugs. Sci. Rep. 2018, 8, 1219. [Google Scholar] [CrossRef] [Green Version]
- Guidetti, G.; Di Cerbo, A.; Giovazzino, A.; Rubino, V.; Palatucci, A.T.; Centenaro, S.; Fraccaroli, E.; Cortese, L.; Bonomo, M.G.; Ruggiero, G.; et al. In Vitro Effects of Some Botanicals with Anti-Inflammatory and Antitoxic Activity. J. Immunol. Res. 2016, 2016, 5457010. [Google Scholar] [CrossRef] [Green Version]
- Gallo, A.; Landi, R.; Rubino, V.; Di Cerbo, A.; Giovazzino, A.; Palatucci, A.T.; Centenaro, S.; Guidetti, G.; Canello, S.; Cortese, L.; et al. Oxytetracycline induces DNA damage and epigenetic changes: A possible risk for human and animal health? PeerJ 2017, 5, e3236. [Google Scholar] [CrossRef] [Green Version]
- Klein, E.; Ben-Bassat, H.; Neumann, H.; Ralph, P.; Zeuthen, J.; Polliack, A.; Vanky, F. Properties of the K562 cell line, derived from a patient with chronic myeloid leukemia. Int. J. Cancer 1976, 18, 421–431. [Google Scholar] [CrossRef]
- Qian, J.; Zhou, C.H.; Qian, Z.; Nan, F.J.; Ye, Q.Z. Development of a K562 cell-based assay for screening anticancer agents. Acta Pharm. Sin. 2001, 22, 821–826. [Google Scholar]
- Zhang, J.; Zhang, Q. Using Seahorse Machine to Measure OCR and ECAR in Cancer Cells. Methods Mol. Biol. 2019, 1928, 353–363. [Google Scholar] [CrossRef] [PubMed]
- Perry, S.W.; Norman, J.P.; Barbieri, J.; Brown, E.B.; Gelbard, H.A. Mitochondrial membrane potential probes and the proton gradient: A practical usage guide. Biotechniques 2011, 50, 98–115. [Google Scholar] [CrossRef] [PubMed]
- Shadel, G.S.; Horvath, T.L. Mitochondrial ROS signaling in organismal homeostasis. Cell 2015, 163, 560–569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, H.S.; Dighe, P.A.; Mezera, V.; Monternier, P.A.; Brand, M.D. Production of superoxide and hydrogen peroxide from specific mitochondrial sites under different bioenergetic conditions. J. Biol. Chem. 2017, 292, 16804–16809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hempel, S.L.; Buettner, G.R.; O’Malley, Y.Q.; Wessels, D.A.; Flaherty, D.M. Dihydrofluorescein diacetate is superior for detecting intracellular oxidants: Comparison with 2’,7’-dichlorodihydrofluorescein diacetate, 5(and 6)-carboxy-2’,7’-dichlorodihydrofluorescein diacetate, and dihydrorhodamine 123. Free Radic. Biol. Med. 1999, 27, 146–159. [Google Scholar] [CrossRef]
- Palikaras, K.; Tavernarakis, N. Mitochondrial homeostasis: The interplay between mitophagy and mitochondrial biogenesis. Exp. Gerontol. 2014, 56, 182–188. [Google Scholar] [CrossRef]
- Scarpulla, R.C. Metabolic control of mitochondrial biogenesis through the PGC-1 family regulatory network. Biochim. Biophys. Acta 2011, 1813, 1269–1278. [Google Scholar] [CrossRef] [Green Version]
- Um, J.H.; Yun, J. Emerging role of mitophagy in human diseases and physiology. Bmb Rep. 2017, 50, 299–307. [Google Scholar] [CrossRef] [Green Version]







| Gene | Product Number |
|---|---|
| PPARGC1A | QT00095578 |
| TFAM | QT00012782 |
| NRF1 | QT01154076 |
| NRF2 | QT01192688 |
| PINK1 | QT00056630 |
| PARK2 | QT00023401 |
| BNIP3L | QT00022939 |
| GAPDH | QT00079247 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pacelli, C.; Di Cerbo, A.; Lecce, L.; Piccoli, C.; Canello, S.; Guidetti, G.; Capitanio, N. Effect of Chicken Bone Extracts on Metabolic and Mitochondrial Functions of K562 Cell Line. Pharmaceuticals 2020, 13, 114. https://doi.org/10.3390/ph13060114
Pacelli C, Di Cerbo A, Lecce L, Piccoli C, Canello S, Guidetti G, Capitanio N. Effect of Chicken Bone Extracts on Metabolic and Mitochondrial Functions of K562 Cell Line. Pharmaceuticals. 2020; 13(6):114. https://doi.org/10.3390/ph13060114
Chicago/Turabian StylePacelli, Consiglia, Alessandro Di Cerbo, Lucia Lecce, Claudia Piccoli, Sergio Canello, Gianandrea Guidetti, and Nazzareno Capitanio. 2020. "Effect of Chicken Bone Extracts on Metabolic and Mitochondrial Functions of K562 Cell Line" Pharmaceuticals 13, no. 6: 114. https://doi.org/10.3390/ph13060114