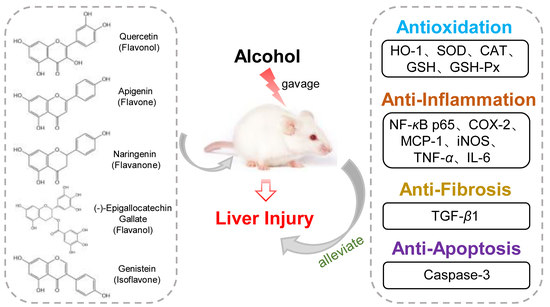
Protective Effects of Five Structurally Diverse Flavonoid Subgroups against Chronic Alcohol-Induced Hepatic Damage in a Mouse Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Animals and Experimental Design
2.3. Determination of Serum Biochemical Parameters
2.4. Determination of Hepatic Biochemical Parameters
2.5. Liver Histopathology
2.6. Statistical Analysis
3. Results
3.1. Effect of Five Kinds of Flavonoids on Food Intake, Body Weight, Liver Weight, and Liver Index in Mice
3.2. Effect of Five Kinds of Flavonoids on Serum Biochemical Markers
3.3. Effect of Five Kinds of Flavonoids on Hepatic Lipid Profiles
3.4. Effect of Five Kinds of Flavonoids on Hepatic Lipid Peroxidation and Oxidative Stress
3.5. Effect of Five Kinds of Flavonoids on Hepatic Inflammatory Stress
3.6. Effect of Five Kinds of Flavonoids on Hepatic Fibrosis and Apoptosis
3.7. Liver Histopathological Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Liangpunsakul, S.; Haber, P.; McCaughan, G.W. Alcoholic Liver Disease in Asia, Europe, and North America. Gastroenterology 2016, 150, 1786–1797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sugimoto, K.; Takei, Y. Pathogenesis of alcoholic liver disease. Hepatol. Res. 2017, 47, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.D.; Lee, S.R.; Kim, T.; Jang, S.-A.; Kang, S.C.; Koo, H.J.; Sohn, E.; Bak, J.P.; Namkoong, S.; Kim, H.K. Fucoidan from fucus vesiculosus protects against alcohol-induced liver damage by modulating inflammatory mediators in mice and Hepg2 cells. Mar. Drugs 2015, 13, 1051–1067. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Status Report on Alcohol and Health 2018. Available online: http://www.who.int/substance_abuse/publications/global_alcohol_report/en/ (accessed on 21 September 2018).
- Orman, E.S.; Odena, G.; Bataller, R. Alcoholic liver disease: Pathogenesis, management, and novel targets for therapy. J. Gastroenterol. Hepatol. 2013, 28, 77–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, L.; Wang, Y.; Liu, J.; Wang, K.; Guo, X.; Ji, B.; Wu, W.; Zhou, F. Protective effects of genistein and puerarin against chronic alcohol-induced liver injury in mice via antioxidant, anti-inflammatory, and anti-apoptotic mechanisms. J. Agric. Food Chem. 2016, 64, 7291–7297. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Zhang, R.; Huang, F.; Liu, L.; Deng, Y.; Ma, Y.; Wei, Z.; Tang, X.; Zhang, Y.; Zhang, M. Lychee (Litchi chinensis Sonn.) pulp phenolic extract confers a protective activity against alcoholic liver disease in mice by alleviating mitochondrial dysfunction. J. Agric. Food Chem. 2017, 65, 5000–5009. [Google Scholar] [CrossRef] [PubMed]
- Wang, O.; Cheng, Q.; Liu, J.; Wang, Y.; Zhao, L.; Zhou, F.; Ji, B.P. Hepatoprotective effect of Schisandra chinensis (Turcz.) Baill. lignans and its formula with Rubus idaeus on chronic alcohol-induced liver injury in mice. Food Funct. 2014, 5, 3018–3025. [Google Scholar] [CrossRef] [PubMed]
- Ross, J.A.; Kasum, C.M. Dietary flavonoids: Bioavailability, metabolic effects, and safety. Annu. Rev. Nutr. 2002, 22, 19–34. [Google Scholar] [CrossRef] [PubMed]
- Jaganath, I.B.; Crozier, A. Dietary Flavonoids and Phenolic Compounds. In Plant Phenolics and Human Health: Biochemistry, Nutrition, and Pharmacology; Wiley: New York, NY, USA, 2010. [Google Scholar]
- Van De Wier, B.; Koek, G.H.; Bast, A.; Haenen, G.R. The potential of flavonoids in the treatment of non-alcoholic fatty liver disease. Crit. Rev. Food Sci. Nutr. 2017, 57, 834–855. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Mateos, A.; Vauzour, D.; Krueger, C.G.; Shanmuganayagam, D.; Reed, J.; Calani, L.; Mena, P.; Del Rio, D.; Crozier, A. Bioavailability, bioactivity and impact on health of dietary flavonoids and related compounds: An update. Arch. Toxicol. 2014, 88, 1803–1853. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Liu, J.C.; Zhou, R.J.; Zhao, X.; Liu, M.; Ye, H.; Xie, M.L. Apigenin protects against alcohol-induced liver injury in mice by regulating hepatic CYP2E1-mediated oxidative stress and PPARalpha-mediated lipogenic gene expression. Chem. Biol. Interact. 2017, 275, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Zhou, X.; Zhao, J. Quercetin prevents alcohol-induced liver injury through targeting of PI3K/Akt/nuclear factor-kappaB and STAT3 signaling pathway. Exp. Ther. Med. 2017, 14, 6169–6175. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Zhou, Z.; Zhong, W.; Huang, P.; Ma, N.; Zhang, Y.; Zhou, C.; Lai, Y.; Huang, S.; An, H.; et al. Naringenin inhibits alcoholic injury by improving lipid metabolism and reducing apoptosis in zebrafish larvae. Oncol. Rep. 2017, 38, 2877–2884. [Google Scholar] [CrossRef] [PubMed]
- Kaviarasan, S.; Sundarapandiyan, R.; Anuradha, C.V. Epigallocatechin gallate, a green tea phytochemical, attenuates alcohol-induced hepatic protein and lipid damage. Toxicol. Mech. Methods 2008, 18, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Hou, W.; Yao, P.; Zhang, B.; Sun, S.; Nüssler, A.K.; Liu, L. Quercetin protects against ethanol-induced oxidative damage in rat primary hepatocytes. Toxicol. In Vitro 2010, 24, 516–522. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Tao, Z.; Jin, Y.; Yuan, Y.; Dong, T.T.X.; Tsim, K.W.K.; Zhou, Z. Flavonoids, a Potential New Insight of Leucaena leucocephala Foliage in Ruminant Health. J. Agric. Food Chem. 2018, 66, 7616–7626. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Wang, L.; Zhang, H.; Zhang, D.; Zhang, Z.; Zhang, J. Protective effect of artemisinin on chronic alcohol induced-liver damage in mice. Environ. Toxicol. Pharmacol. 2017, 52, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Koneru, M.; Sahu, B.D.; Kumar, J.M.; Kuncha, M.; Kadari, A.; Kilari, E.K.; Sistla, R. Fisetin protects liver from binge alcohol-induced toxicity by mechanisms including inhibition of matrix metalloproteinases (MMPs) and oxidative stress. J. Funct. Foods 2016, 22, 588–601. [Google Scholar] [CrossRef]
- Huang, Q.; Huang, R.; Zhang, S.; Lin, J.; Wei, L.; He, M.; Zhuo, L.; Lin, X. Protective effect of genistein isolated from Hydrocotyle sibthorpioides on hepatic injury and fibrosis induced by chronic alcohol in rats. Toxicol. Lett. 2013, 217, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Jayaraman, J.; Jesudoss, V.A.; Menon, V.P.; Namasivayam, N. Anti-inflammatory role of naringenin in rats with ethanol induced liver injury. Toxicol. Mech. Methods 2012, 22, 568–576. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Gao, C.; Xing, M.; Li, Y.; Zhu, L.; Wang, D.; Yang, X.; Liu, L.; Yao, P. Quercetin prevents ethanol-induced dyslipidemia and mitochondrial oxidative damage. Food Chem. Toxicol. 2012, 50, 1194–1200. [Google Scholar] [CrossRef] [PubMed]
- Yuan, G.J.; Gong, Z.J.; Zhou, X.R.; Zhang, P.; Sun, X.M.; Li, X. Epigallocatechin-3-gallate ameliorates alcohol-induced liver injury in rats. Int. J. Mol. Sci. 2006, 7, 204–219. [Google Scholar] [CrossRef]
- Tang, C.-C.; Lin, W.-L.; Lee, Y.-J.; Tang, Y.-C.; Wang, C.-J. Polyphenol-rich extract of Nelumbo nucifera leaves inhibits alcohol-induced steatohepatitis via reducing hepatic lipid accumulation and anti-inflammation in C57BL/6J mice. Food Funct. 2014, 5, 678–687. [Google Scholar] [CrossRef] [PubMed]
- Louvet, A.; Mathurin, P. Alcoholic liver disease: Mechanisms of injury and targeted treatment. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 231–242. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.L.; Tai, S.Y.; Chen, J.W.; Chou, C.H.; Fu, S.G.; Chen, Y.C. Ameliorative effects of pepsin-digested chicken liver hydrolysates on development of alcoholic fatty livers in mice. Food Funct. 2017, 8, 1763–1774. [Google Scholar] [CrossRef] [PubMed]
- Jayachitra, J.; Nalini, N. Effect of naringenin (citrus flavanone) on lipid profile in ethanol-induced toxicity in rats. J. Food Biochem. 2012, 36, 502–511. [Google Scholar] [CrossRef]
- Kaviarasan, S.; Viswanathan, P.; Ravichandran, M.K.; Anuradha, C.V. (-) Epigallocatechin gallate (EGCG) prevents lipid changes and collagen abnormalities in chronic ethanol-fed rats. Toxicol. Mech. Methods 2008, 18, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, Y.; Bai, R.; Wang, M.; Du, S. Baicalin attenuates alcoholic liver injury through modulation of hepatic oxidative stress, inflammation and sonic hedgehog pathway in rats. Cell. Physiol. Biochem. 2016, 39, 1129–1140. [Google Scholar] [CrossRef] [PubMed]
- Ye, H.; Qi, X.; Jiang, H.; Xin, H.; Wei, L.; Li, Z. Maltol, a food flavoring agent, attenuates acute alcohol-induced oxidative damage in mice. Nutrients 2015, 7, 682–696. [Google Scholar] [CrossRef]
- Vidhya, A.; Indira, M. Protective effect of Quercetin in the Regression of Ethanol-Induced Hepatotoxicity. Indian J. Pharm. Sci. 2009, 71, 527–532. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Xie, L.; Jia, G.; Cai, S.; Ji, B.; Liu, Y.; Wu, W.; Zhou, F.; Wang, A.; Chu, L.; et al. Comparative study on antioxidant capacity of flavonoids and their inhibitory effects on oleic acid-induced hepatic steatosis in vitro. Eur. J. Med. Chem. 2011, 46, 4548–4558. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Jin, X.; Li, Q.; Sawaya, A.C.H.F.; Le Leu, R.K.; Conlon, M.A.; Wu, L.; Hu, F. Propolis from different geographic origins decreases intestinal inflammation and Bacteroides spp. Populations in a model of DSS-induced colitis. Mol. Nutr. Food Res. 2018, 62, 1800080. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, W.; Hu, H.; Tang, N.; Zhang, C.; Liang, W.; Wang, M. Smad3 specific inhibitor, naringenin, decreases the expression of extracellular matrix induced by TGF-beta1 in cultured rat hepatic stellate cells. Pharm. Res. 2006, 23, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Gao, B.; Bataller, R. Alcoholic liver disease: Pathogenesis and new therapeutic targets. Gastroenterology 2011, 141, 1572–1585. [Google Scholar] [CrossRef] [PubMed]
- Jang, M.H.; Shin, M.C.; Shin, H.S.; Kim, K.H.; Park, H.J.; Kim, E.H.; Kim, C.J. Alcohol induces apoptosis in TM3 mouse Leydig cells via bax-dependent caspase-3 activation. Eur. J. Pharmacol. 2002, 449, 39–45. [Google Scholar] [CrossRef]
- Choi, Y.; Abdelmegeed, M.A.; Song, B.J. Preventive effects of indole-3-carbinol against alcohol-induced liver injury in mice via antioxidant, anti-inflammatory, and anti-apoptotic mechanisms: Role of gut-liver-adipose tissue axis. J. Nutr. Biochem. 2018, 55, 12–25. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Wang, J.; Xing, F.; Han, T.; Jiao, R.; Liong, E.C.; Fung, M.-L.; So, K.-F.; Tipoe, G.L. Zeaxanthin dipalmitate therapeutically improves hepatic functions in an alcoholic fatty liver disease model through modulating MAPK pathway. PLoS ONE 2014, 9, e95214. [Google Scholar] [CrossRef] [PubMed] [Green Version]








| Variables | NG | MG | Que | Api | Nar | EGCG | Gen |
|---|---|---|---|---|---|---|---|
| AST (U/L) | 142 ± 12.29 a | 274 ± 21.94 f | 208 ± 13.13 e | 167 ± 10.68 bc | 195 ± 17.91 de | 182 ± 16.40 cd | 160 ± 13.15 b |
| ALT (U/L) | 30.0 ± 4.14 a | 56.0 ± 3.73 d | 40.3 ± 2.79 bc | 37.6 ± 2.56 b | 37.2 ± 1.85 b | 40.1 ± 1.93 bc | 42.6 ± 6.56 c |
| HDL-C (mmol/L) | 1.55 ± 0.12 d | 0.72 ± 0.08 a | 1.28 ± 0.24 b | 1.21 ± 0.12 b | 1.36 ± 0.14 bc | 1.53 ± 0.10 cd | 1.37 ± 0.16 bcd |
| LDL-C (mmol/L) | 0.976 ± 0.15 a | 2.12 ± 0.21 e | 1.43 ± 0.17 cd | 1.08 ± 0.12 ab | 1.45 ± 0.26 cd | 1.24 ± 0.17 bc | 1.55 ± 0.15 d |
| serum TG (mmol/L) | 1.24 ± 0.14 a | 1.81 ± 0.04 c | 1.57 ± 0.13 b | 1.42 ± 0.21 ab | 1.47 ± 0.24 b | 1.40 ± 0.17 ab | 1.55 ± 0.27 b |
| serum TC (mmol/L) | 3.39 ± 0.17 a | 5.05 ± 0.44 c | 4.75 ± 0.31 bc | 4.52 ± 0.22 b | 4.48 ± 0.20 b | 4.78 ± 0.34 bc | 4.58 ± 0.18 b |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, L.; Zhang, N.; Yang, D.; Yang, M.; Guo, X.; He, J.; Wu, W.; Ji, B.; Cheng, Q.; Zhou, F. Protective Effects of Five Structurally Diverse Flavonoid Subgroups against Chronic Alcohol-Induced Hepatic Damage in a Mouse Model. Nutrients 2018, 10, 1754. https://doi.org/10.3390/nu10111754
Zhao L, Zhang N, Yang D, Yang M, Guo X, He J, Wu W, Ji B, Cheng Q, Zhou F. Protective Effects of Five Structurally Diverse Flavonoid Subgroups against Chronic Alcohol-Induced Hepatic Damage in a Mouse Model. Nutrients. 2018; 10(11):1754. https://doi.org/10.3390/nu10111754
Chicago/Turabian StyleZhao, Liang, Nanhai Zhang, Dong Yang, Mengyan Yang, Xiaoxuan Guo, Jiguo He, Wei Wu, Baoping Ji, Qian Cheng, and Feng Zhou. 2018. "Protective Effects of Five Structurally Diverse Flavonoid Subgroups against Chronic Alcohol-Induced Hepatic Damage in a Mouse Model" Nutrients 10, no. 11: 1754. https://doi.org/10.3390/nu10111754