Fabrication of Bimetal CuFe2O4 Oxide Redox-Active Nanocatalyst for Oxidation of Pinene to Renewable Aroma Oxygenates
Abstract
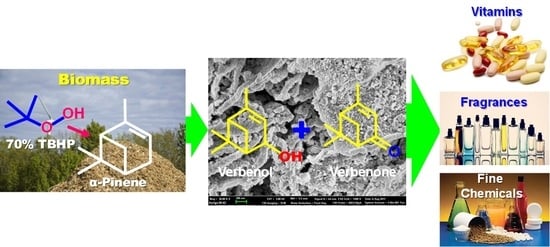
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Catalyst Preparation
2.3. Catalyst Characterization Procedures
2.4. Catalytic Activity Testing
2.4.1. Atmospheric Pressure Pinene Oxidation Reactions
2.4.2. High-Pressure Batch Reactor Oxidation Reactions
2.4.3. Analysis of Pinene Oxidation Products
3. Results
3.1. Catalyst Characterization
3.2. Catalytic Oxidation of Pinene
3.2.1. Catalytic Activity Screening of CuFe2O4 Catalysts
3.2.2. Effect of Different Reaction Parameters on Pinene Oxidation Rates
3.2.3. CuFe2O4 Performance under Atmospheric and High-Pressure Conditions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bicas, J.L.; Dionísio, A.P.; Pastore, G.M. Bio-oxidation of terpenes: An approach for the flavour industry. Chem. Rev. 2009, 109, 4518–4531. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, J.L.F.; Veloso, C.O. Catalytic conversion of terpenes into fine chemicals. Top. Catal. 2004, 27, 169–180. [Google Scholar] [CrossRef]
- Schwab, W.; Fuchs, C.; Huang, F.-C. Transformation of terpenes into fine chemicals. Eur. J. Lipid Sci. Technol. 2013, 115, 3–8. [Google Scholar] [CrossRef]
- Mäki-Arvela, P.; Holmbom, B.; Salmi, T.; Murzin, D.Y. Recent progress in synthesis of fine and specialty chemicals from wood and other biomass by heterogeneous catalytic processes. Catal. Rev. 2007, 49, 197–340. [Google Scholar] [CrossRef]
- Golets, M.; Ajaikumar, M.S.; Mikkola, J.P. Catalytic upgrading of extractives to chemicals: Monoterpenes to “EXICALS”. Chem. Rev. 2015, 115, 3141–3169. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.-C.; Yang, W.-J.; Mao, Y.-L. Selectively aerobic oxidation of C-C and allylic C-H bonds in α-pinene over simple metalloporphyrins. J. Mol. Catal. A Chem. 2005, 226, 279–284. [Google Scholar] [CrossRef]
- Maksimchuk, N.V.; Melgunov, M.S.; Mrowiec-Białoń, J.; Jarzębski, A.B.; Kholdeeva, O.A. H2O2-based allylic oxidation of α-pinene over different single site catalysts. J. Catal. 2005, 235, 175–183. [Google Scholar] [CrossRef]
- Patil, M.V.; Yadav, M.K.; Jasra, R.V. Catalytic epoxidation of α-pinene with molecular oxygen using cobalt(II)-exchanged zeolite Y-based heterogeneous catalysts. J. Mol. Catal. A Chem. 2007, 277, 72–80. [Google Scholar] [CrossRef]
- Raupp, Y.S.; Yildiz, C.; Kleist, W.; Meier, M.A.R. Aerobic oxidation of α-pinene catalyzed by homogeneous and MOF-based Mn catalysts. Appl. Catal. A Gen. 2017, 546, 1–6. [Google Scholar] [CrossRef]
- Ajaikumar, S.; Ahlkvist, J.; Larsson, W.; Shchukarev, A.; Mikkola, J.-P. Oxidation of α-pinene over gold containing bimetallic nanoparticles supported on reducible TiO2 by deposition-precipitation method. Appl. Catal. A Gen. 2011, 392, 11–18. [Google Scholar] [CrossRef]
- Rauchdi, M.; Ali, M.A.; Roucoux, A.; Denicourt-Nowicki, A. Novel access to verbenone via ruthenium nanoparticles-catalyzed oxidation of α-pinene in neat water. Appl. Catal. A Gen. 2018, 550, 266–273. [Google Scholar] [CrossRef]
- Casuscelli, S.G.; Eimer, G.A.; Canepa, A.; Heredia, A.C. Ti-MCM-41 as catalyst for pinene oxidation: Study of the effect of Ti content and H2O2 addition on activity and selectivity. Catal. Today 2008, 133, 678–683. [Google Scholar] [CrossRef]
- Caovilla, M.; Caovilla, A.; Pergher, S.B.C.; Esmelindro, M.C. Catalytic oxidation of limonene, α-pinene and β-pinene by the complex [Fe3+(BPMP)Cl(μ-O)Fe3+Cl3] biomimetic to MMO enzyme. Catal. Today 2008, 133, 669–698. [Google Scholar]
- Rachwalik, R.; Hunger, M.; Sulikowski, B. Transformations of monoterpene hydrocarbons on ferrierite type zeolites. Appl. Catal. A Gen. 2012, 427–428, 98–105. [Google Scholar] [CrossRef]
- Capoute, M.; Peeters, J.; Noziere, B.; Muller, J.F. Alpha pinene oxidation by OH: Simulations of laboratory experiments. Atmos. Chem. Phys. 2004, 4, 2285–2311. [Google Scholar] [CrossRef]
- Romanenko, E.P.; Taraban, E.A.; Tkachev, A.V. Catalytic oxidation of α-pinene with tert-butyl hydroperoxide in the presence pf Fe-pillared montmolillonite. Russian Chem. Bull. 2006, 55, 993–998. [Google Scholar] [CrossRef]
- Lajanen, M.K. Co(II) catalyzed oxidation of α-pinene by molecular oxygen part III. J. Mol. Catal. A Chem. 2001, 169, 33–40. [Google Scholar] [CrossRef]
- Cánepa, A.L.; Chanquía, C.M.; Vaschetti, V.M.; Eimer, G.A.; Casuscelli, S.G. Biomass toward fine chemical products: Oxidation of α-pinene over sieves nanostructured modified with vanadium. J. Mol. Catal. A Chem. 2015, 404–405, 65–73. [Google Scholar] [CrossRef]
- Martínez, H.; Amaya, Á.A.; Páez-Mozo, E.A.; Martínez, O.F. Highly efficient epoxidation of α-pinene with O2 photocatalyzed by dioxoMo(VI) complex anchored on TiO2 nanotubes. Microp. Mesop. Mater. 2018, 265, 202–210. [Google Scholar] [CrossRef]
- Timofeeva, M.N.; Hasan, Z.; Panchenko, V.N.; Prosvirin, I.P.; Jhung, S.H. Vanadium-containing nickel phosphate molecular sieves as catalysts for α-pinene oxidation with molecular oxygen: A study of the effect of vanadium content on activity and selectivity. J. Mol. Catal. A Chem. 2012, 363–364, 328–334. [Google Scholar] [CrossRef]
- Parida, K.M.; Rath, D. Structural properties and catalytic oxidation of benzene to phenol over CuO-impregnated mesoporous silica. Appl. Catal. A Gen. 2007, 321, 101–108. [Google Scholar] [CrossRef]
- Sun, L.; Liu, J.; Luo, W.; Yang, Y.; Suib, S.L. Preparation of amorphous copper–chromium oxides catalysts for selective oxidation of cyclohexane. Mol. Catal. 2018, 460, 16–26. [Google Scholar] [CrossRef]
- Bhat, P.B.; Rajarao, R.; Sahajwalla, V.; Bhat, B.R. Immobilized magnetic nano catalyst for oxidation of alcohol. J. Mol. Catal. A Chem. 2015, 409, 42–49. [Google Scholar] [CrossRef]
- Balasubramanyan, S.; Arayathody, S.; Sugunan, S.; Narayanan, B.N. Selective liquid-phase oxidation of cyclohexene over magnetic Fe3O4/graphene oxide nanocomposite. Mater Chem. Phys. 2018, 211, 23–33. [Google Scholar] [CrossRef]
- Sun, N.; Zhang, X.; Jin, L.; Hu, B.; Hu, X. Recyclable copper-catalyzed ambient aerobic oxidation of primary alcohols to aldehydes in water using water-soluble PEG-functionalized pyridine triazole as ligand. Catal. Commun. 2017, 101, 5–9. [Google Scholar] [CrossRef]
- Feng, X.; Lv, P.; Sun, W.; Han, X.; Zheng, G. Reduced graphene oxide-supported Cu nanoparticles for the selective oxidation of benzyl alcohol to aldehyde with molecular oxygen. Catal. Commun. 2017, 99, 105–109. [Google Scholar] [CrossRef]
- Munoz, M.; de Pedro, Z.M.; Casas, J.A.; Rodriguez, J.J. Preparation of magnetite-based catalysts and their application in heterogeneous Fenton oxidation—A review. Appl. Catal. B Environ. 2015, 176–177, 249–265. [Google Scholar] [CrossRef]
- Teja, A.S.; Koh, P.-Y. Synthesis, properties, and applications of magnetic iron oxide nanoparticles. Prog. Cryst. Growth Charact. Mater. 2009, 55, 22–45. [Google Scholar] [CrossRef]
- Cornell, R.M.; Schwertmann, U. The Iron Oxides: Structure, Properties, Reactions, Occurrences and Uses; Willey-VCH GmbH & Co., KGaA: Weinheim, Germany, 2003; ISBN 3-527-30274-3. [Google Scholar]
- Wu, L.; Mendoza-Garcia, A.; Li, Q.; Sun, S. Organic phase syntheses of magnetic nanoparticles and their applications. Chem. Rev. 2016, 116, 10473–10512. [Google Scholar] [CrossRef]
- Arumugam, V.; Sriram, P.; Yen, T.-J.; Redhi, G.G.; Gengan, R.M. Nano-material as an excellent catalyst for reducing a series of nitroanilines and dyes: Triphosphonated ionic liquid-CuFe2O4-modified boron nitride. Appl. Catal. B Environ. 2018, 222, 99–114. [Google Scholar] [CrossRef]
- Makgwane, P.R.; Ray, S.S. Hydroxylation of benzene to phenol over magnetic recyclable nanostructured CuFe mixed-oxide catalyst. J. Mol. Catal. A Chem. 2015, 398, 149–157. [Google Scholar] [CrossRef]
- Zhao, Y.; He, G.; Dai, W.; Chen, H. High catalytic activity in the phenol hydroxylation of magnetically separable CuFe2O4-reduced graphene oxide. Ind. Eng. Chem. Res. 2014, 53, 12566–12574. [Google Scholar] [CrossRef]
- Gao, W.; Li, S.; Huo, H.; Li, F.; Li, R. Investigation of the crystal structure of Cu-Fe bimetal oxide and their catalytic activity for the Baeyer-Villiger oxidation reaction. Mol. Catal. 2017, 439, 108–117. [Google Scholar] [CrossRef]
- Rasal, K.B.; Yadav, G.D. One-pot synthesis of benzimidazole using DMF as a multitasking reagent in presence CuFe2O4 as catalyst. Catal. Today 2018, 309, 51–60. [Google Scholar] [CrossRef]
- Yu, D.; Ni, H.; Wang, L.; Wu, M.; Yang, X. Nanoscale-confined precursor of CuFe2O4 mediated by hyperbranched polyamide as an unusual heterogeneous Fenton catalyst for efficient dye degradation. J. Clean. Prod. 2018, 186, 146–154. [Google Scholar] [CrossRef]
- Zhuang, Y.-T.; Gao, W.; Yu, Y.-L.; Wang, J.-H. Facile fabrication of three-dimensional porous CuFe2O4 cages as highly efficient and recyclable heterogeneous catalyst. Mater. Des. 2017, 130, 294–301. [Google Scholar] [CrossRef]
- Lei, Y.; Chen, C.-S.; Tu, Y.-J.; Huang, Y.-H.; Zhang, H. Heterogeneous degradation of organic pollutants by persulfate activated by CuO-Fe3O4: Mechanism, stability, and effects of pH and bicarbonate ions. Environ. Sci. Technol. 2015, 49, 6838–6845. [Google Scholar] [CrossRef]
- Tang, Y.; Shih, K.; Liu, C.; Liao, C. Cubic and tetragonal ferrite crystal structures for copper ion immobilization in iron-rich ceramic matrix. R. Soc. Chem. 2015, 125, 145–149. [Google Scholar] [CrossRef]
- Rai, N.K.; Lakshmanna, A.Y.; Namboodiri, V.V.; Umapathy, S. Basic principles of ultrafast Raman loss spectroscopy. J. Chem. Sci. 2012, 124, 177–186. [Google Scholar] [CrossRef]
- Klein, T.; Buhr, E.; Georg Frase, C. 6 TSEM: A Review of scanning electron Microscopy in transmission mode and its applications. Adv. Imaging Electron Phys. 2012, 171, 297. [Google Scholar]
- Shifeier, L. Analytical Study of Osteoporosis of Maxilla in Ovariectomized Rats. Master’s Thesis, Queensland University Of Technology, Brisbane, Australia, 2015. ID Code 88474. [Google Scholar]
- Arnal, C.; Alfe, M.; Gargiulo, V.; Ciajolo, A.; Alzueta, M.U.; Millera, A.; Bilbao, R. Characterization of Soot. Cleaner Combustion; Springer: London, UK, 2013; pp. 333–362. [Google Scholar]
- Briggman, K.L.; Bock, D.D. Volume electron microscopy for neuronal circuit reconstruction. Curr. Opin. Neurobiol. 2012, 22, 154–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bogner, A.; Jouneau, P.H.; Thollet, G.; Basset, D.; Gauthier, C. A history of scanning electron microscopy developments: Towards “wet-STEM” imaging. Micron 2007, 38, 390–401. [Google Scholar] [CrossRef] [PubMed]
- Gawade, A.B.; Nakhate, A.V.; Yadav, G.D. Selective synthesis of 2, 5-furandicarboxylic acid by oxidation of 5-hydroxymethylfurfural over MnFe2O4 catalyst. Catal. Today 2018, 309, 119–125. [Google Scholar] [CrossRef]











| Entry | Catalyst | BET SA (m2/g) | V/p (cm3/g) | D/p (nm) |
|---|---|---|---|---|
| 1 | CuO | 2.63 | 0.022 | 47.54 |
| 2 | Fe2O3 | 48.66 | 0.154 | 14.73 |
| 3 | CuFe-1 | 32.38 | 0.065 | 16.85 |
| 4 | CuFe-2 | 30.25 | 0.096 | 12.15 |
| 5 | CuFe-3 | 27.59 | 0.126 | 17.85 |
| 6 | CuFe-4 | 44.66 | 0.186 | 16.44 |
| Entry | Catalysts | Conv. (%) Pinene | Selectivity (%) | |||
|---|---|---|---|---|---|---|
| Pinene Oxide | Verbenol | Verbenone | Others | |||
| 1 | Blank | 3.1 | 55.0 | 9.1 | 6.3 | 29.6 |
| 2 | CuO | 17.1 | 60.3 | 12.3 | 8.2 | 19.2 |
| 3 | Fe2O3 | 10.3 | 23.4 | 17.3 | 38.4 | 20.9 |
| 4 | CuFe-1 | 31.0 | 24.1 | 35.2 | 16.4 | 24.3 |
| 5 | CuFe-2 | 41.7 | 18.7 | 37.1 | 22.2 | 22.0 |
| 6 | CuFe-3 | 35.3 | 14.3 | 36.9 | 23.5 | 25.3 |
| 7 | CuFe-4 | 46.6 | 10.2 | 29.4 | 38.9 | 21.5 |
| Solvent | Conv. (%) Pinene | Selectivity (%) | |||
|---|---|---|---|---|---|
| Pinene Oxide | Verbenol | Verbenone | Others | ||
| Solventless | 17.9 | 41.6 | 14.1 | 7.2 | 37.1 |
| Ethanol | 23.7 | 45.3 | 16.3 | 12.3 | 26.1 |
| DMF | 35.4 | 12.4 | 28.2 | 33.1 | 26.3 |
| THF | 28.3 | 34.8 | 19.1 | 12.4 | 33.7 |
| Acetonitrile | 46.6 | 10.2 | 29.4 | 38.9 | 21.5 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mdletshe, L.S.; Makgwane, P.R.; Ray, S.S. Fabrication of Bimetal CuFe2O4 Oxide Redox-Active Nanocatalyst for Oxidation of Pinene to Renewable Aroma Oxygenates. Nanomaterials 2019, 9, 1140. https://doi.org/10.3390/nano9081140
Mdletshe LS, Makgwane PR, Ray SS. Fabrication of Bimetal CuFe2O4 Oxide Redox-Active Nanocatalyst for Oxidation of Pinene to Renewable Aroma Oxygenates. Nanomaterials. 2019; 9(8):1140. https://doi.org/10.3390/nano9081140
Chicago/Turabian StyleMdletshe, Lindokuhle S., Peter R. Makgwane, and Suprakas S. Ray. 2019. "Fabrication of Bimetal CuFe2O4 Oxide Redox-Active Nanocatalyst for Oxidation of Pinene to Renewable Aroma Oxygenates" Nanomaterials 9, no. 8: 1140. https://doi.org/10.3390/nano9081140