1. Introduction
Malaria is the primary tropical disease in the world. It causes nearly 300 million clinical malaria cases and half a million deaths annually [
1]. Malaria prevention and elimination programs face challenges using traditional vector control methods since resistance to the main classes of insecticides has spread to all vectors in all malaria-endemic countries [
2]. Furthermore, the
Plasmodium parasites have developed resistance against the most commonly used antimalarial drugs throughout Africa and Asia [
3], especially amid concerns about the spread of artemisinin resistance over the last five years. This process may accelerate in the future [
4,
5]. The international malaria community urges new strategies and next-generation drugs to control malaria.
To discover new drug leads, screening structurally diverse synthetic or natural small-molecule libraries with high-throughput screening (HTS) led to identifying several novel antimalarial compounds [
6]. Among these screening methods,
Plasmodium falciparum growth assays with cultured parasites are relatively robust [
7], and most of these screenings target asexual stage malaria. Transmission-blocking small molecules were rarely reported because transmission-blocking reagents need to focus either on the sexual stage of parasites or malaria vectors. In contrast, disease transmission molecular mechanisms are not well understood.
To our knowledge, HTS against FREP1-
Plasmodium interaction is the only approach targeting malaria transmission [
8]. The target protein, fibrinogen-related protein 1 (FREP1) from
Anopheles gambiae, is critical for parasites to infect the mosquito midgut [
9] through its interaction with gametocytes or ookinetes [
10]. This conserved pathway exists across multiple species of
Plasmodium and
Anopheles [
11]. Therefore, the drugs discovered by this approach are potentially functional for controlling all malaria pathogens and vectors to spread malaria.
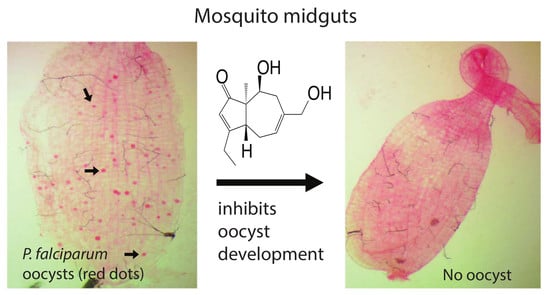
This study screened a fungal extract library (Global Fungal Extract Library, GFEL) and obtained a candidate extract that completely blocked
P. falciparum transmission to
An. gambiae at 100 µg/mL. Further assays identified the fungal species as
Aspergillus aculeatus. Many bioactive secondary metabolites have been isolated from this fungal species [
12,
13]. The active compound was isolated and determined to be asperaculane B. The structure of asperaculane B has resolved previously [
12,
14]. Notably, asperaculane B also inhibited asexual
P. falciparum infection in blood while being nontoxic to human cells. Therefore, asperaculane B reported in this paper is a promising lead in developing a drug that treats malaria patients as well as blocks the spread of malaria.
3. Discussion
The international malaria communities are challenged by the fast spread of insecticide-resistant mosquitoes and drug-resistant Plasmodium parasites, as well as difficulties in vaccine development. It makes the control even more difficult since a patient under the treatment of conventional antimalarial drugs can still transmit malaria before he/she completely recovers. Therefore, novel drugs that combine the treatment of malaria patients and malaria transmission prevention are essential for malaria control.
Plasmodium falciparum growth assays are used widely to screen drugs against sexual stage malaria, e.g., traditional antimalaria drugs to control blood-stage parasites [
7]. It is urgently needed to identify potential drugs that cut the malaria transmission cycles. The newly discovered FREP1-mediated
Plasmodium transmission pathway [
10] provided us with an ideal target to block malaria transmission. Since FREP1 plays its function through binding to parasite-infected cells, screening small molecules through ELISA that inhibit the interaction is an optimal high throughput assay [
8]. We demonstrated this approach by screening our newly constructed global fungal extract library and discovered an
A. aculeatus isolate, GFEL-46E5, that effectively inhibited
P. falciparum transmission to mosquitoes.
A. aculeatus belongs to an
Aspergillus fungal subgroup, section
Nigri (the black aspergilli), which consists of 18 species [
16]. Several drug candidates against tumors or bacteria, e.g., okaramines H and I, have been isolated from this group [
15]. However, some of
A. aculeatus species also release mycotoxins [
17,
18]. The
P-orlandin from
Aspergillus niger, another member of this section, has been isolated, and it significantly inhibits
P. falciparum transmission to mosquitoes [
8].
The active compound from
A. aculeatus strain GFEL-46E5 was identified as asperaculane B, a sesquiterpenoid. This paper is the first to report that asperaculane B has antimalarial activity. Notably, asperaculane B inhibited both the sexual stage and asexual stage
P. falciparum, which should benefit public health communities in controlling malaria substantially. Asperaculane B inhibits the interaction between FREP1 and parasites, which may be responsible for its activity of blocking malaria transmission. The molecular mechanism of asperaculane B against blood-stage malaria is unknown. Asperaculane B has been shown to have nontoxicity to human cancer cells and regular hamster cell lines even at the concentration of 50 µM [
12], much higher than its IC
50 against malaria parasites. Here, we demonstrated that asperaculane B had no cytotoxic effects on HEK293 cells at the concentrations of 120 μM. Therefore, the impact of asperaculane B against sexual and asexual
P. falciparum is not through general cytotoxicity.
As a natural product, asperaculane B has some advantages for future drug development. Firstly,
Aspergillus fungi have been used as a resource for drug discovery. For example, aspergillusol A is an α-glucosidase inhibitor and works as an oral anti-diabetic drug [
19], and aculeacins A–G have antifungal and antibiotic activities [
20]. Therefore, many features are known, and related technologies are ready. Secondly, a large amount of fungal secondary metabolites can be obtained by large-scale fermentation. Moreover, the recent development of genomic sequencing technology and the identification of more biosynthetic gene clusters speed up the process of mass production of the active fungal metabolites and significantly reduce the cost of mass production of this compound. Thirdly, this compound did not show toxicity to human and mammalian cells [
12], and it is not a mycotoxin. Finally, its derivatives or other druggable features, and the accumulated experience of total synthesis of sesquiterpenoid compounds from academia and industry should help accelerate the process of drug development [
21]. Collectively, these features make asperaculane B an excellent antimalarial and transmission-blocking drug candidate for future development.
4. Materials and Methods
4.1. Maintenance of An. Gambiae Mosquitoes
The mosquitoes (An. gambiae G3 strain) were maintained in an insectary room maintained at 27 °C, 80% humidity with a 12-h day/night cycle. Adults were fed on 8% sucrose and fed with mouse blood for egg production, and larvae were fed daily with 0.1 mg grounded fish food.
4.2. Screening a Fungal Extract Library with an ELISA Method
Our research group recently constructed GFEL. GFEL contains about 10,000 isolates from soil or plants collected worldwide. Each fungal species was grown on cereal-based medium at 25 ± 2 °C for four weeks to produce metabolites. The ethyl acetate (Thermo Fisher Scientific, Waltham, MA, USA) was used to extract small molecules, and the dry extracts were dissolved in dimethyl sulfoxide (DMSO) (Thermo Fisher Scientific, Waltham, MA, USA) at the concentration of 10 mg/mL stock. In vitro, high throughput screening was based on the ELISA method described in detail previously [
8]. First, we coated 100 μL 15-day old cultured
P. falciparum-infected cell lysates (2mg/mL proteins in PBS containing 0.2% Tween-20) for one hour at RT (room temperature). One microliter of each crude extract with a concentration of 2 mg/mL in DMSO was mixed with 99 μL FREP1 proteins in PBS (1:99) to determine whether an extract can disrupt the interaction of FREP1 and
P. falciparum lysate. The fungal extracts that reduced the ELISA signals (A
405) of FREP1 binding to
P. falciparum-infected red blood cell lysates were considered to contain active compounds. The mixture containing 1% DMSO was a negative control.
4.3. Mass Production and Extraction of Fungal Metabolites
The fungal isolates were cultured in liquid malt extract medium (MEA) (Life Tech, Grand Island, NY, USA) for 3-5 days and transferred to one autoclaved mushroom bags containing 550mL of sterile and dried Cheerios cereal (General Mills, Minneapolis, MN, USA) and 980 mL of 0.3% sucrose supplemented with 50 mg/L chloramphenicol (Life Tech, Grand Island, NY, USA) at 25 ± 2 °C for four weeks. The fungal metabolites were extracted with one litter of ethyl acetate twice, and the organic layer was filtered with the two layers of cheesecloth in the Buchner funnel. The resultant ethyl acetate supernatant was dried using a rotary evaporator (Heidolph, Elk Grove Village, IL, USA).
4.4. Transmission-Blocking Assays
P. falciparum was maintained with RPMI-1640 medium (Life Tech, Grand Island, NY) containing 10% heat-inactivated human AB+ serum (Interstate blood bank, Memphis, TN), 12.5 μg/mL hypoxanthine and 4% hematocrit (O+ human blood) in a candle jar at 37 °C for 15–17 days. The medium was replaced every day. Parasitemia or gametocytaemia were monitored every other day under a light microscope using Giemsa-stained blood smear.
The 15–17-day old P. falciparum cultures containing 2–3% gametocytes at stage V were collected and diluted with new O+ type human blood, and mixed with the same volume of heat-inactivated AB+ human serum. Then, the candidate fungal extract, fractions, or pure compounds in DMSO were mixed with the infected blood (the final DMSO concentration in blood was <1%). SMFA was performed to feed ~100 3–5 days old female mosquitoes for 30 m. The midguts were dissected seven days post-infection and stained with 0.1% mercury dibromofluresein disodium salt (Sigma-Aldrich, St. Louis, MO, USA) in PBS. The oocysts were counted under light microscopy.
4.5. Determination of Fungal Species
The fungal isolate was grown in liquid MEA at 25 ± 2 °C for three days, and the collected fungi were used for DNA extraction. The genomic DNA applied as a PCR template was isolated using DNAzol Reagent from the collected fungi following the manual (Life Tech). Next, the nuclear ribosomal internal transcribed spacer (ITS) region was amplified with the primer set of ITS1F (5′-CTTGGTCATTTAGAGGAAGTAA-3′) and ITS4 (5′-TCCTCCGCTTATTGATATGC-3′) primers and Phusion DNA polymerase (Thermo Fisher Scientific) with the method as the following: 98 °C 30 s; 98 °C 15 s, 55 °C 15 s, 72 °C 15 s, 35 cycles; 72 °C 5 m. The amplified products were sequenced, and the sequences were blasted against the NCBI database for the identification of the fungal species.
4.6. Purification of the Active Compounds by Gravity Chromatography and High-Performance Liquid Chromatography
The solvents used for extraction and separation were all from Thermo Fisher Scientific if not explicitly mentioned. The crude extract (1 g) was loaded into a column with the diameter and length of the column of 2.5 cm and 30 cm, respectively. The fine silica used for column chromatography was of particle size 40–63 µm (60 × 100 mesh) (Sigma Aldrich, St. Louis, MO, USA). Then, the column was eluted with 200 mL of hexane, followed by elution sequentially with 400 mL of 50% ethyl acetate in hexane, 500 mL of 5% methanol in dichloromethane and 300 mL of 100% methanol. All four fractions were dried, and their activities in blocking malaria transmission were determined as described above. The fractions containing active compounds against malaria transmission were further fractioned using a Shimadzu HPLC system (an LC-20AD pump, SPD-M20A UV and visible detector, and FRC-10A fraction collector, Shimadzu, Columbia, MD, USA). A semi-purification column (Gemini C18 250 mm × 10 mm, 5 μm, Phenomenex, Torrance, CA, USA) and a gradient solvent of MeOH-H2O (50:50–100:0) was used to obtain the pure bioactive compound.
4.7. LC-MS and High-Resolution Mass Spectrum
LC-MS includes Agilent 1100 series LC system consisting of G1312B binary pump, G1313A autosampler and 1200 Series diode-array detector (Agilent Technologies, Santa Clara, CA, USA) with an analytic column (Hypersil GOLD aQ, 3 µm C18, 150 x 2.1 mm) (Thermo Fisher Scientific) run on a gradient solvent of MeOH-H2O (50:50–100:0) at a flow rate of 0.2 mL/m. The eluted compounds were then analyzed with mass spectrometry using (+) ESI mode on the Agilent 6220 TOF mass spectrometer (Gas temperature - 350˚C, Drying gas (N2)–8 L/m. Nebulizer–2 Bar).
High-resolution mass spectrometry was recorded using (+) ESI mode on the Bruker Daltonics, Impact II QTOF mass spectrometer (Gas temperature −200 °C, Drying gas (N2)–4 L/m Nebulizer–0.3 Bar, Bruker Scientific LLC, Billerica, MA, USA). Both LC-MS and high-resolution mass spectrometry analyses were conducted at the University of Florida Mass Spectrometry Research and Education Center, Department of Chemistry.
4.8. Asexual Plasmodium Falciparum Growth Inhibition Assays
P. falciparum-infected human red blood cells were mixed with new AB+ type uninfected human RBCs to prepare cultures with 0.5% parasitemia and 2% hematocrit. The compound was dissolved in DMSO at the concentration of 1mg/mL and diluted to various concentrations with DMSO. About 2 μL of small molecule solution was added into 1 mL of culture per well in a 24-well plate. The plate was incubated in a candle jar at 37 °C. The medium, as well as the candidate compound, was replaced at 48 h. The parasitemia was recorded at 24 h, 48 h, 72 h, and 96 h after incubation. IC50 was determined by analysis of the dose-response curve made by GraphPad Prism (GraphPad Software, CA, USA).
4.9. Cytotoxic Assay
The MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide) cell proliferation assay (Thermo Fisher Scientific) was used to measure the general cytotoxicity of pure compounds to human embryonic kidney 293 (HEK293) cell line. The HEK293 cells at a concentration of 2 × 105 cells/well in 100 μL culture medium containing different levels of the pure compounds, e.g., 10–400 µM)] were seeded into microplates and incubated for 24 h at 37 °C and 5% CO2. Next, the cells were incubated with 100µL of MTT reagent for four h at 37°C (final concentration 0.5 mg/mL). After removing all but 25 µL of the medium from the wells, 100 µL of DMSO was added to each well and incubated for 10 m to dissolve the purple formazan crystal for measurement. The optical density at an absorbance wavelength of 540 nm was measured with the plate reader.
4.10. Statistical Analysis
All the experiments were independently repeated at least twice. As reported previously [
8,
9,
10], the number of oocysts in mosquito midguts does not follow normal distribution. Therefore, nonparametric statistical analysis, the Wilcoxon–Mann–Whitney test, were used to calculate the
p-value of infection difference between control and experimental groups in each assay with Prism (GraphPad Software, CA, USA).