Sorbitol as a Polar Pharmacological Modifier to Enhance the Hydrophilicity of 99mTc-Tricarbonyl-Based Radiopharmaceuticals
Abstract
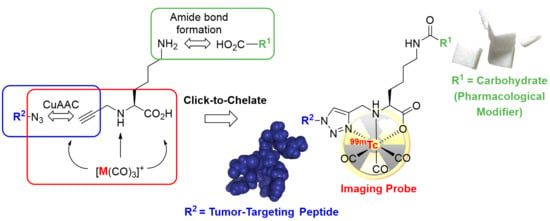
:1. Introduction
2. Results and Discussion
2.1. Syntheses
2.2. (Radio) Metal-Labeling and Characterization
2.3. TLC Analytics
2.4. LogD
2.5. In Vitro Evaluation
2.6. In Vivo Evaluation
3. Materials and Methods
3.1. General Procedures
3.2. Syntheses
3.3. Radiolabeling
3.3.1. [99mTc][Tc(CO)3(H2O)3]+
3.3.2. [99mTc][Tc(CO)3(L)] (L = 12, 13)
3.3.3. [natRe(CO)3(L)] (L = 12, 13)
3.4. TLC
3.5. LogD
3.6. In Vitro Evaluation
3.6.1. General Methods
3.6.2. Internalization Studies
3.6.3. Receptor Saturation Assay
3.7. In Vivo Evaluation
Biodistribution Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rösch, F.; Baum, R.P. Generator-based PET radiopharmaceuticals for molecular imaging of tumours: On the way to THERANOSTICS. Dalt. Trans. 2011, 40, 6104–6111. [Google Scholar] [CrossRef] [PubMed]
- Brandt, M.; Cardinale, J.; Aulsebrook, M.L.; Gasser, G.; Mindt, T.L. An Overview of PET Radiochemistry, Part 2: Radiometals. J. Nucl. Med. 2018, 59, 1500–1506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- García-Garayoa, E.; Schibli, R.; Schubiger, A.P. Peptides radiolabeled with Re-186/188 and Tc-99m as potential diagnostic and therapeutic agents. Nucl. Sci. Tech. 2007, 18, 88–100. [Google Scholar] [CrossRef]
- Boschi, A.; Uccelli, L.; Martini, P. A picture of modern Tc-99m radiopharmaceuticals: Production, chemistry, and applications in molecular imaging. Appl. Sci. 2019, 9, 2526. [Google Scholar] [CrossRef] [Green Version]
- Alberto, R.; Ortner, K.; Wheatley, N.; Schibli, R.; Schubiger, A.P. Synthesis and properties of boranocarbonate: A convenient in situ CO source for the aqueous preparation of [99mTc(OH2)3(CO)3]+. J. Am. Chem. Soc. 2001, 123, 3135–3136. [Google Scholar] [CrossRef] [PubMed]
- Kluba, C.A.; Mindt, T.L. Click-to-Chelate: Development of technetium and rhenium-tricarbonyl labeled radiopharmaceuticals. Molecules 2013, 18, 3206–3226. [Google Scholar] [CrossRef] [PubMed]
- Kasten, B.B.; Ma, X.; Liu, H.; Hayes, T.R.; Barnes, C.L.; Qi, S.; Cheng, K.; Bottorff, S.C.; Slocumb, W.S.; Wang, J.; et al. Clickable, hydrophilic ligand for fac-[MI(CO)3] + (M = Re/99mTc) applied in an S-functionalized α-MSH peptide. Bioconjug. Chem. 2014, 25, 579–592. [Google Scholar] [CrossRef]
- García-Garayoa, E.; Schweinsberg, C.; Maes, V.; Brans, L.; Bläuenstein, P.; Tourwé, D.A.; Schibli, R.; Schubiger, A.P. Influence of the molecular charge on the biodistribution of bombesin analogues labeled with the [99mTc(CO)3]-core. Bioconjug. Chem. 2008, 19, 2409–2416. [Google Scholar] [CrossRef]
- Mindt, T.L.; Schweinsberg, C.; Brans, L.; Hagenbach, A.; Abram, U.; Tourwé, D.; García-Garayoa, E.; Schibli, R. A click approach to structurally diverse conjugates containing a central di-1,2,3-triazole metal chelate. Chem. Med. Chem. 2009. [CrossRef]
- Däpp, S.; García-Garayoa, E.; Maes, V.; Brans, L.; Tourwé, D.A.; Müller, C.; Schibli, R. PEGylation of 99mTc-labeled bombesin analogues improves their pharmacokinetic properties. Nucl. Med. Biol. 2011, 38, 997–1009. [Google Scholar] [CrossRef]
- Valverde, I.E.; Vomstein, S.; Fischer, C.A.; Mascarin, A.; Mindt, T.L. Probing the backbone function of tumor targeting peptides by an amide-to-triazole substitution strategy. J. Med. Chem. 2015. [CrossRef] [PubMed]
- Moradi, S.V.; Hussein, W.M.; Varamini, P.; Simerska, P.; Toth, I. Glycosylation, an effective synthetic strategy to improve the bioavailability of therapeutic peptides. Chem. Sci. 2016, 7, 2492–2500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schottelius, M.; Laufer, B.; Kessler, H.; Wester, H.J. Ligands for mapping αvβ3-integrin expression in vivo. Acc. Chem. Res. 2009, 42, 969–980. [Google Scholar] [CrossRef] [PubMed]
- Mindt, T.L.; Struthers, H.; Brans, L.; Anguelov, T.; Schweinsberg, C.; Maes, V.; Tourwe, D.; Schibli, R. “Click to chelate”: Synthesis and installation of metal chelates into biomolecules in a single step. J. Am. Chem. Soc. 2006, 128, 15096–15097. [Google Scholar] [CrossRef]
- Rostovtsev, V.V.; Green, L.G.; Fokin, V.V.; Sharpless, K.B. A stepwise huisgen cycloaddition process: Copper(I)-catalyzed regioselective “ligation” of azides and terminal alkynes. Angew. Chem. Int. Ed. 2002, 41, 2596–2599. [Google Scholar] [CrossRef]
- Tornøe, C.W.; Christensen, C.; Meldal, M. Peptidotriazoles on solid phase: [1,2,3]-triazoles by regiospecific copper(I)-catalyzed 1,3-dipolar cycloadditions of terminal alkynes to azides. J. Org. Chem. 2002, 67, 3057–3064. [Google Scholar] [CrossRef]
- Mindt, T.L.; Struthers, H.; Spingler, B.; Brans, L.; Tourwé, D.; García-Garayoa, E.; Schibli, R. Molecular assembly of multifunctional 99mTc radiopharmaceuticals using “clickable” amino acid derivatives. Chem. Med. Chem. 2010, 5, 2026–2038. [Google Scholar] [CrossRef]
- Römhild, K.; Fischer, C.A.; Mindt, T.L. Glycated 99mTc-tricarbonyl-labeled peptide conjugates for tumor targeting by “click-to-chelate”. Chem. Med. Chem. 2017, 12, 66–74. [Google Scholar] [CrossRef]
- Rangger, C.; Haubner, R. Radiolabelled peptides for positron emission tomography and endoradiotherapy in oncology. Pharmaceuticals 2020, 13, 22. [Google Scholar] [CrossRef] [Green Version]
- Weinstein, E.A.; Ordonez, A.A.; DeMarco, V.P.; Murawski, A.M.; Pokkali, S.; MacDonald, E.M.; Klunk, M.; Mease, R.C.; Pomper, M.G.; Jain, S.K. Imaging enterobacteriaceae infection in vivo with 18F-fluorodeoxysorbitol positron emission tomography. Sci. Transl. Med. 2014, 6, 259ra146. [Google Scholar] [CrossRef] [Green Version]
- Leamon, C.P.; Reddy, J.A.; Klein, P.J.; Vlahov, I.R.; Dorton, R.; Bloomfield, A.; Nelson, M.; Westrick, E.; Parker, N.; Bruna, K.; et al. Reducing undesirable hepatic clearance of a tumor-targeted vinca alkaloid via novel saccharopeptidic modifications. J. Pharm. Exp. 2011, 336, 336–343. [Google Scholar] [CrossRef] [Green Version]
- Jagadeesh, Y.; Reddy, J.S.; Rao, V.B.; Swarnalatha, L.J. Total synthesis of (−)-codonopsinol and (+)-2-epi codonopsinol via acid catalyzed amido cyclisation. Tetrahedron 2010, 66, 1202–1207. [Google Scholar] [CrossRef]
- Kluba, C.A.; Bauman, A.; Valverde, I.E.; Vomstein, S.; Mindt, T.L. Dual-targeting conjugates designed to improve the efficacy of radiolabeled peptides. Org. Biomol. Chem. 2012, 10, 7594–7602. [Google Scholar] [CrossRef]
- Alberto, R.; Egli, A.; Abram, U.; Hegetschweiler, K.; Gramlich, V.; Schubiger, A.P. Synthesis and reactivity of [NEt4]2[ReBr3(CO)3]. Formation and structural characterization of the clusters [NEt4][Re3(µ3-OH)(µ-OH)3(CO)9] and [NEt4][Re2(µ-OH)3(CO)6] by alkaline titration. J. Chem. Soc. Dalt. Trans. 1994, 2815–2820. [Google Scholar] [CrossRef]
- Vraka, C.; Nics, L.; Wagner, K.H.; Hacker, M.; Wadsak, W.; Mitterhauser, M. LogP, a yesterday’s value? Nucl. Med. Biol. 2017, 50, 1–10. [Google Scholar] [CrossRef]
- OECD. Test No. 117: Partition Coefficient (n-Octanol/Water), HPLC Method; OECD publishing: Paris, France, 2004. [Google Scholar] [CrossRef]
- Fischer, C.A.; Vomstein, S.; Mindt, T.L. A bombesin-shepherdin radioconjugate designed for combined extra- and intracellular targeting. Pharmaceuticals 2014. [Google Scholar] [CrossRef] [Green Version]
- Schweinsberg, C.; Maes, V.; Brans, L.; Bläuenstein, P.; Tourwé, D.A.; Schubiger, A.P.; Schibli, R.; García-Garayoa, E. Novel glycated [99mTc(CO)3]-labeled bombesin analogues for improved targeting of gastrin-releasing peptide receptor-positive tumors. Bioconjug. Chem. 2008, 19, 2432–2439. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |






| Compound[a] | TLC | HPLC | |
|---|---|---|---|
| Rf | Radiochemical Purity (%) | Radiochemical Purity (%) | |
| [99mTc]TcO4- | 1.0 | 100 | 100 |
| [99mTc][Tc(CO)3(H2O)3]+ | 0.0 | >95 | >95 |
| [99mTc][Tc(CO)3(13)] | 0.30–0.35 | 94.4 ± 0.2 | 95 ± 0.1 |
| [99mTc][Tc(CO)3(12)] | 0.30–0.35 | 93.1 ± 0.5 | 94 ± 0.2 |
| Compound[a] | Total Cell Binding and Internalization % AD/106 Cells[b] | Kd [nM][c] | Bmax [nM][c] | logD[d] |
|---|---|---|---|---|
| [99mTc][Tc(CO)3(13)] | 25.0 ± 0.1 | 3.7 ± 1.0 | 0.37 ± 0.02 | −0.54 ± 0.04 |
| [99mTc][Tc(CO)3(12)] | 22.9 ± 0.4 | 21.3 ± 4.6 | 0.91 ± 0.06 | −1.26 ± 0.16 |
| Organ | [99mTc][Tc(CO)3(13)] (% ID/g)[b] | [99mTc][Tc(CO)3(12)] (% ID/g)[b] | ||
|---|---|---|---|---|
| 1 h | 1 h Blocked | 1 h | 1 h Blocked | |
| Adrenal[a] | 1.85 ± 0.67 | 0.46 ± 0.32 | 5.34 ± 1.85 | 0.54 ± 0.20 |
| Blood | 0.98 ± 0.14 | 1.03 ± 0.41 | 1.22 ± 0.40 | 1.24 ± 0.34 |
| Bone | 0.31 ± 0.07 | 0.52 ± 0.15 | 0.44 ± 0.28 | 0.63 ± 0.50 |
| Brain | 0.05 ± 0.01 | 0.06 ± 0.02 | 0.09 ± 0.07 | 0.07 ± 0.04 |
| Colon[a] | 3.40 ± 1.31 | 0.39 ± 0.18 | 5.85 ± 1.29 | 0.34 ± 0.11 |
| Heart | 0.32 ± 0.02 | 0.36 ± 0.14 | 0.50 ± 0.23 | 0.57 ± 0.12 |
| Kidney | 3.58 ± 0.70 | 3.79 ± 1.22 | 4.32 ± 0.97 | 3.64 ± 0.95 |
| Intestine[a] | 3.66 ± 1.60 | 1.11 ± 0.07 | 3.71 ± 1.48 | 0.69 ± 0.32 |
| Liver | 3.31 ± 0.60 | 3.19 ± 0.72 | 2.75 ± 0.74 | 2.85 ± 0.86 |
| Lung | 0.97 ± 0.30 | 1.03 ± 0.38 | 1.38 ± 0.83 | 1.69 ± 0.69 |
| Muscle | 0.30 ± 0.28 | 1.94 ± 1.08 | 0.35 ± 0.24 | 0.29 ± 0.04 |
| Pancreas[a] | 9.31 ± 1.84 | 0.52 ± 0.13 | 17.06 ± 3.25 | 0.60 ± 0.20 |
| Spleen | 1.16 ± 0.18 | 0.64 ± 0.13 | 1.16 ± 0.17 | 0.79 ± 0.32 |
| Stomach[a] | 3.79 ± 1.53 | 1.32 ± 0.77 | 3.03 ± 0.88 | 0.62 ± 0.35 |
| Tumor[a] | 2.43 ± 0.49 | 0.68 ± 0.17 | 2.72 ± 0.88 | 0.67 ± 0.14 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giammei, C.; Balber, T.; Benčurová, K.; Cardinale, J.; Berroterán-Infante, N.; Brandt, M.; Jouini, N.; Hacker, M.; Mitterhauser, M.; Mindt, T.L. Sorbitol as a Polar Pharmacological Modifier to Enhance the Hydrophilicity of 99mTc-Tricarbonyl-Based Radiopharmaceuticals. Molecules 2020, 25, 2680. https://doi.org/10.3390/molecules25112680
Giammei C, Balber T, Benčurová K, Cardinale J, Berroterán-Infante N, Brandt M, Jouini N, Hacker M, Mitterhauser M, Mindt TL. Sorbitol as a Polar Pharmacological Modifier to Enhance the Hydrophilicity of 99mTc-Tricarbonyl-Based Radiopharmaceuticals. Molecules. 2020; 25(11):2680. https://doi.org/10.3390/molecules25112680
Chicago/Turabian StyleGiammei, Carolina, Theresa Balber, Katarina Benčurová, Jens Cardinale, Neydher Berroterán-Infante, Marie Brandt, Nedra Jouini, Marcus Hacker, Markus Mitterhauser, and Thomas L. Mindt. 2020. "Sorbitol as a Polar Pharmacological Modifier to Enhance the Hydrophilicity of 99mTc-Tricarbonyl-Based Radiopharmaceuticals" Molecules 25, no. 11: 2680. https://doi.org/10.3390/molecules25112680