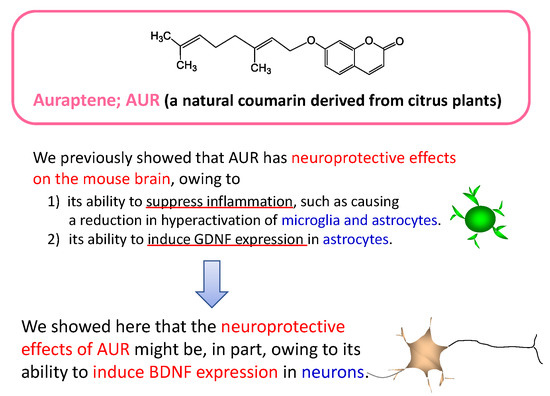
Citrus Auraptene Induces Expression of Brain-Derived Neurotrophic Factor in Neuro2a Cells
Abstract
:1. Introduction
2. Results
2.1. Effects of AUR on the Viability of Neuro2a Cells
2.2. Effects of AUR on BDNF mRNA Expression in Neuro2a Cells
2.3. Effects of AUR on BDNF Content of Medium Conditioned by Neuro2a Cells
2.4. Effects of MEK Inhibitor on AUR-Induced BDNF mRNA Expression in Neuro2a Cells
3. Discussion
4. Materials and Methods
4.1. Chemical and Reagents
4.2. Cell Culture
4.3. Determination of Cell Viability
4.4. Total RNA Extraction and RT-PCR
4.5. ELISA
4.6. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Genovese, S.; Epifano, F. Auraptene: A natural biologically active compound with multiple targets. Curr. Drug Targets 2011, 12, 381–386. [Google Scholar] [CrossRef] [PubMed]
- Epifano, F.; Molinaro, G.; Genovese, S.; Ngomba, R.T.; Nicoletti, F.; Curini, M. Neuroprotective effect of prenyloxycoumarins from edible vegetables. Neurosci. Lett. 2008, 443, 57–60. [Google Scholar] [CrossRef] [PubMed]
- Okuyama, S.; Minami, S.; Shimada, N.; Makihata, M.; Nakajima, M.; Furukawa, Y. Anti-inflammatory and neuroprotective effect of auraptene, a citrus coumarin, following cerebral global ischemia in mice. Eur. J. Pharmacol. 2013, 699, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Okuyama, S.; Morita, M.; Kaji, M.; Amakura, Y.; Yoshimura, M.; Shimamoto, K.; Ookido, Y.; Nakajima, M.; Furukawa, Y. Auraptene acts as an anti-inflammatory agent in the mouse brain. Molecules 2015, 20, 20230–20239. [Google Scholar] [CrossRef] [Green Version]
- Okuyama, S.; Yamamoto, K.; Mori, H.; Toyoda, N.; Yoshimura, M.; Amakura, Y.; Yoshida, T.; Sugawara, K.; Sudo, M.; Nakajima, M.; et al. Auraptene in the peels of Citrus kawachiensis (Kawachi Bankan) ameliorates lipopolysaccharide (LPS)-induced inflammation in the mouse brain. Evid.-Based Complement. Altern. Med. 2014. [Google Scholar] [CrossRef] [Green Version]
- Okuyama, S.; Semba, T.; Toyoda, N.; Epifano, F.; Genovese, S.; Fiorito, S.; Taddeo, V.A.; Sawamoto, A.; Nakajima, M.; Furukawa, Y. Auraptene and other prenyloxyphenylpropanoids suppress microglial activation and dopaminergic neuronal cell death in a lipopolysaccharide-induced model of Parkinson’s disease. Int. J. Mol. Sci. 2016, 17, 1716. [Google Scholar] [CrossRef] [Green Version]
- Furukawa, Y.; Okuyama, S.; Amakura, S.; Watanabe, S.; Fukata, T.; Nakajima, M.; Yoshimura, M.; Yoshida, T. Isolation and characterization of activators of ERK/MAPK from Citrus plants. Int. J. Mol. Sci. 2012, 13, 1832–1845. [Google Scholar] [CrossRef]
- Furukawa, Y.; Watanabe, S.; Okuyama, S.; Nakajima, M. Neurotrophic effect of citrus auraptene: Neuritegenic activity in PC12 cells. Int. J. Mol. Sci. 2012, 13, 5338–5347. [Google Scholar] [CrossRef]
- Furukawa, Y.; Hara, R.; Nakaya, M.; Okuyama, S.; Sawamoto, A.; Nakajima, M. Citrus auraptene induces glial cell line-derived neurotrophic factor in C6 cells. Int. J. Mol. Sci. 2019, 21, 253. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Weingarten, D.P.; Callahan, J.W.; Sachar, K.; deVellis, J. Regulation of mRNAs for three enzymes in the glial cell model C6 cell line. J. Neurochem. 1984, 43, 1455–1463. [Google Scholar] [CrossRef]
- Huang, E.J.; Reichardt, L.F. Neurotrophins: Roles in neuronal development and function. Annu. Rev. Neurosci. 2001, 24, 677–736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sawamoto, A.; Okuyama, S.; Nakajima, M.; Furukawa, Y. Citrus flavonoid 3,5,6,7,8,3′,4′-heptamethoxy- flavone induces BDNF via cAMP/ERK/CREB signaling and reduces phosphodiesterase activity in C6 cells. Pharmacol. Rep. 2019, 71, 653–658. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Chopp, M. Astrocytes, therapeutic targets for neuroprotection and neurorestoration in ischemic stroke. Prog. Neurobiol. 2016, 144, 103–120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, B.; Teschemacher, A.G.; Kasparov, S. Astroglia as a cellular target for neuroprotection and treatment of neuro-psychiatric disorders. Glia 2017, 65, 1205–1226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matias, I.; Buosi, A.S.; Gomes, F.C. Functions of flavonoids in the central nervous system: Astrocytes as targets for natural compounds. Neurochem. Int. 2016, 95, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Toyomoto, M.; Inoue, S.; Ohta, K.; Kuno, S.; Ohta, M.; Hayashi, K.; Ikeda, K. Production of NGF, BDNF and GDNF in mouse astrocyte cultures is strongly enhanced by a cerebral vasodilator, ifenprodil. Neurosci. Lett. 2005, 379, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Lindholm, D.; Dechant, G.; Heisenberg, C.P.; Thoenen, H. Brain-derived neurotrophic factor is a survival factor for cultured rat cerebellar granule neurons and protects them against glutamate-induced neurotoxicity. Eur. J. Neurosci. 1993, 5, 1455–1464. [Google Scholar] [CrossRef]
- Panja, D.; Bramham, C.R. BDNF mechanisms in late LTP formation: A synthesis and breakdown. Neuropharmacology 2014, 76 Pt C, 664–676. [Google Scholar] [CrossRef]
- Chen, S.D.; Wu, C.L.; Hwang, W.C.; Yang, D.I. More insight into BDNF against neurodegeneration: Anti-apoptosis, anti-oxidation, and suppression of autophagy. Int. J. Mol. Sci. 2017, 18, 545. [Google Scholar] [CrossRef] [Green Version]
- Lu, B.; Nagappan, G.; Guan, X.; Nathan, P.J.; Wren, P. BDNF-based synaptic repair as a disease-modifying strategy for neurodegenerative diseases. Nat. Rev. Neurosci. 2013, 14, 401–416. [Google Scholar] [CrossRef]
- Amakura, Y.; Yoshimura, M.; Ouchi, K.; Okuyama, S.; Furukawa, Y.; Yoshida, T. Characterization of constituents in peel of Citrus kawachiensis (Kawachibankan). Biosci. Biotechnol. Biochem. 2013, 77, 1977–1980. [Google Scholar] [CrossRef] [PubMed]
- Igase, M.; Okada, Y.; Ochi, M.; Igase, K.; Ochi, H.; Okuyama, S.; Furukawa, Y.; Ohyagi, Y. Auraptene in the peels of Citrus Kawachiensis (Kawachibankan) contributes to the preservation of cognitive function: A randomized, placebo-controlled, double-blind study in healthy volunteers. J. Prev. Alzheimers Dis. 2017, 3, 1–5. [Google Scholar] [CrossRef]
- Okuyama, S.; Kotani, Y.; Yamamoto, K.; Sawamoto, A.; Sugawara, K.; Sudo, M.; Ohkubo, Y.; Tamanaha, A.; Nakajima, M.; Furukawa, Y. The peel of Citrus kawachiensis (Kawachi Bankan) ameliorates microglial activation, tau hyper-phosphorylation, and suppression of neurogenesis in the hippocampus of senescence-accelerated mice. Biosci. Biotechnol. Biochem. 2018, 82, 869–878. [Google Scholar] [CrossRef] [PubMed]
- Okuyama, S.; Yamamoto, K.; Mori, H.; Sawamoto, A.; Amakura, A.; Yoshimura, M.; Sugawara, K.; Sudo, M.; Nakajima, M.; Furukawa, Y. Neuroprotective effect of Citrus kawachiensis (Kawachi Bankan) peels, a rich source of naringin, against global cerebral ischemia/reperfusion injury in mice. Biosci. Biotechnol. Biochem. 2018, 82, 1216–1224. [Google Scholar] [CrossRef]
- Okuyama, S.; Shinoka, W.; Nakamura, K.; Kotani, M.; Sawamoto, A.; Sugawara, K.; Sudo, M.; Nakajima, M.; Furukawa, Y. Suppressive effects of the peel of Citrus kawachiensis (Kawachi Bankan) on astroglial activation, tau phosphorylation, and inhibition of neurogenesis in the hippocampus of type 2 diabetic db/db mice. Biosci. Biotechnol. Biochem. 2018, 82, 1384–1395. [Google Scholar] [CrossRef]
- Okuyama, S.; Katoh, M.; Kanzaki, T.; Kotani, Y.; Amakura, A.; Yoshimura, M.; Fukuda, N.; Tamai, T.; Sawamoto, A.; Nakajima, M.; et al. Auraptene/naringin-rich fruit juice of Citrus kawachiensis (Kawachi bankan) prevents ischemia-induced neuronal cell death in mouse brain through anti-inflammatory responses. J. Nutr. Sci. Vitaminol. 2019, 65, 66–71. [Google Scholar] [CrossRef] [Green Version]
- Okuyama, S.; Kanzaki, T.; Kotani, Y.; Katoh, M.; Sawamoto, A.; Nakajima, M.; Furukawa, Y. Continual treatment with the peels of Citrus kawachiensis (Kawachi Bankan) protects the dopaminergic neuronal cell death in a lipopolysaccharide-induced model of Parkinson’s disease. J. Nutr. Sci. Vitaminol. 2019, 65, 205–208. [Google Scholar] [CrossRef]
- Furukawa, Y.; Watanabe, S.; Okuyama, S.; Amakura, Y.; Yoshimura, M.; Yoshida, T.; Nakajima, M. Effect of citrus polymethoxyfavones on neuritogenesis in neuroblastoma cells. Biointerface Res. Applied Chem. 2012, 2, 432–437. [Google Scholar]
- Furukawa, Y.; Sawamoto, A.; Yamaoka, M.; Nakaya, M.; Hieda, Y.; Choshi, T.; Hatae, N.; Okuyama, S.; Nakajima, M.; Hibino, S. Effects of carbazole derivatives on neurite outgrowth and hydrogen peroxide- induced cytotoxicity in neuro2a cells. Molecules 2019, 24, 1366. [Google Scholar] [CrossRef] [Green Version]
Sample Availability: Samples are not available from the authors. |




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Furukawa, Y.; Washimi, Y.-s.; Hara, R.-i.; Yamaoka, M.; Okuyama, S.; Sawamoto, A.; Nakajima, M. Citrus Auraptene Induces Expression of Brain-Derived Neurotrophic Factor in Neuro2a Cells. Molecules 2020, 25, 1117. https://doi.org/10.3390/molecules25051117
Furukawa Y, Washimi Y-s, Hara R-i, Yamaoka M, Okuyama S, Sawamoto A, Nakajima M. Citrus Auraptene Induces Expression of Brain-Derived Neurotrophic Factor in Neuro2a Cells. Molecules. 2020; 25(5):1117. https://doi.org/10.3390/molecules25051117
Chicago/Turabian StyleFurukawa, Yoshiko, Yu-suke Washimi, Ryu-ichi Hara, Mizuki Yamaoka, Satoshi Okuyama, Atsushi Sawamoto, and Mitsunari Nakajima. 2020. "Citrus Auraptene Induces Expression of Brain-Derived Neurotrophic Factor in Neuro2a Cells" Molecules 25, no. 5: 1117. https://doi.org/10.3390/molecules25051117