The Sustained Delivery of Resveratrol or a Defined Grape Powder Inhibits New Blood Vessel Formation in a Mouse Model of Choroidal Neovascularization
Abstract
:1. Introduction
2. Results and Discussion
2.1. Adlibitum Intake of Water Containing either Resveratrol or Reconstituted Grape Powder


2.2. Bolus Delivery of Resveratrol by Daily Oral Gavage

2.3. Bolus Delivery of Reconstituted Grape Powder by Daily Oral Gavage

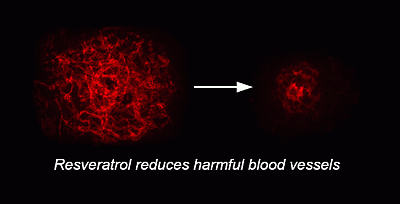
2.4. Continuous Delivery of Resveratrol through a Slow Release Osmotic Pump

2.5. Resveratrol Causes Dose- and Time-Dependent Growth Inhibition of Choroidal Endothelial Cells in Vitro

2.6. Resveratrol Activates p53 in Choroidal Endothelial Cells

2.7. Resveratrol Inhibits the Migration of Choroidal Endothelial Cells in Vitro

2.8. Resveratrol Inhibits Akt/Protein Kinase B in Choroidal Endothelial Cells

2.9. Resveratrol Inhibits Choroidal Endothelial Cell Tube Formation as an in Vitro Correlate of Angiogenesis

2.10. Resveratrol Increases Cytoplasmic Calcium in Activated Endothelial Cells


2.11. Discussion
3. Experimental Section
3.1. Materials
3.2. Treatment Groups
3.3. Implantation of Alzet Pumps
3.4. Laser-Induced CNV
3.5. Statistical Analysis of Animal Studies
3.6. In Vitro Cell Viability
3.7. Western Blot Analysis
3.8. Cell Migration Assay
3.9. Capillary Morphogenesis Assay
3.10. Calcium Imaging
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Eye Diseases Prevalence Research Group. Prevalence of age related macular degeneration in the United States. Arch. Ophthalmol. 2004, 122, 564–572. [Google Scholar]
- Ambati, J.; Ambati, B.K.; Yoo, S.H.; Lanchulev, S.; Adamis, A.P. Age-related macular degeneration: Etiology, pathogenesis, and therapeutic strategies. Surv. Ophthalmol. 2003, 48, 257–293. [Google Scholar] [CrossRef]
- Jager, R.D.; Mieler, W.F.; Miller, J.W. Age-related macular degeneration. N. Engl. J. Med. 2008, 358, 2606–2617. [Google Scholar] [CrossRef]
- Cheung, L.K.; Eaton, A. Age-Related Macular Degeneration. Pharmacotherapy 2013, 33, 838–855. [Google Scholar] [CrossRef]
- Brown, D.M.; Michels, M.; Kaiser, P.K.; Heier, J.S.; Sy, J.P.; Lanchulev, T. Ranibizumab versus verteporfin photodynamic therapy for neovascular age-related macular degeneration: Two-year results of the ANCHOR study. Ophthalmology 2009, 116, 57–65. [Google Scholar] [CrossRef]
- Sivaprasad, S.; Hykin, P. What is new in the management of wet age-related macular degeneration? Br. Med. Bull. 2013, 105, 201–211. [Google Scholar] [CrossRef]
- Bressler, N.M. Antiangiogenic approaches to age-related macular degeneration today. Ophthalmology 2009, 116, S15–S23. [Google Scholar] [CrossRef]
- Yamazaki, T.; Koizumi, H.; Yamagishi, T.; Kinoshita, S. Subfoveal choroidal thickness after ranibizumab therapy for neovascular age-related macular degeneration: 12-month results. Ophthalmology 2012, 119, 1621–1627. [Google Scholar] [CrossRef]
- Martin, D.F.; Maguire, M.G.; Fine, S.L.; Ying, G.S.; Jaffe, G.J.; Grunwald, J.E.; Toth, C.; Redford, M.; Ferris, F.L. Ranibizumab and bevacizumab for treatment of neovascular age-related macular degeneration: Two-year results. Ophthalmology 2012, 119, 1388–1398. [Google Scholar] [CrossRef]
- Fintak, D.R.; Shah, G.K.; Blinder, K.J.; Regillo, C.D.; Pollack, J.; Heier, J.S.; Hollands, H.; Sharma, S. Incidence of endophthalmitis related to intravitreal injection of bevacizumab and ranibizumab. Retina 2008, 28, 1395–1399. [Google Scholar] [CrossRef]
- Hoevenaars, N.E.; Gans, D.; Missotten, T.; van Rooij, J.; Lesaffre, E.; van Meurs, J.C. Suspected bacterial endophthalmitis following intravitreal anti-VEGF injection: Case series and literature review. Ophthalmologica 2012, 228, 143–147. [Google Scholar] [CrossRef]
- HaHarikumar, K.B.; Aggrawal, B.B. Resveratrol: A multitargeted agent for age-associated chronic diseases. Cell Cycle 2008, 7, 1020–1035. [Google Scholar] [CrossRef]
- Shakibaei, M.; Harikumar, K.B.; Aggrawal, B.B. Resveratrol addiction: To die or not to die. Mol. Nutr. Food Res. 2009, 53, 115–128. [Google Scholar] [CrossRef]
- Kopp, P. Resveratrol, a phytoestrogen found in red wine. A possible explanation for the conundrum of the “French paradox”? Eur. J. Endocrinol. 1998, 138, 619–620. [Google Scholar] [CrossRef]
- Renaud, S.; de Lorgeril, M. Wine, alcohol, platelets, and the French paradox for coronary heart disease. Lancet 1992, 339, 1523–1526. [Google Scholar] [CrossRef]
- Ray, P.S.; Maulik, G.; Cordis, G.A.; Bertelli, A.A.; Bertelli, A.; Das, D.K. The red wine antioxidant resveratrol protects isolated rat hearts from ischemia reperfusion injury. Free Radic. Biol. Med. 1999, 27, 160–169. [Google Scholar] [CrossRef]
- Lekli, I.; Szabo, G.; Juhasz, B.; Das, S.; Das, M.; Varga, E.; Szendrei, L.; Gesztelyi, R.; Varadi, J.; Bak, I.; et al. Protective mechanism of resveratrol against ischemia-reperfusion-induced damage in hearts obtained from Zucker obese rats: The role of GLUT-4 and endothelin. Am. J. Physiol. Heart Circ. Physiol. 2008, 294, H859–H866. [Google Scholar] [CrossRef]
- Dong, W.; Li, N.; Gao, D.; Zhen, H.; Zhang, X.; Li, F. Resveratrol attenuates ischemic brain damage in the delayed phase after stroke and induces messenger RNA and protein express for angiogenic factors. J. Vasc. Surg. 2008, 48, 709–714. [Google Scholar] [CrossRef]
- Sakata, Y.; Zhuang, H.; Kwansa, H.; Koehler, R.C.; Dore, S. Resveratrol protects against experimental stroke: Putative neuroprotective role of heme oxygenase 1. Exp. Neurol. 2010, 224, 325–329. [Google Scholar] [CrossRef]
- Shin, J.A.; Lee, H.; Lim, Y.K.; Koh, Y.; Choi, J.H.; Park, E.M. Therapeutic effects of resveratrol during acute periods following experimental ischemic stroke. J. Neuroimmunol. 2010, 227, 93–100. [Google Scholar] [CrossRef]
- Sun, A.Y.; Wang, Q.; Simonyi, A.; Sun, G.Y. Resveratrol as a therapeutic agent for neurodegenerative diseases. Mol. Neurobiol. 2010, 41, 375–383. [Google Scholar] [CrossRef]
- Aggrawal, B.B.; Bhardwaj, A.; Aggrawal, R.S.; Seeram, N.P.; Shishodia, S.; Takada, Y. Role of resveratrol in prevention and therapy of cancer: Preclinical and clinical studies. Anticancer Res. 2004, 24, 2783–2840. [Google Scholar]
- Jang, M.; Cai, L.; Udeani, G.O.; Slowing, K.V.; Thomas, C.F.; Beecher, C.W.; Fong, H.H.; Farnsworth, N.R.; Kinghorn, A.D.; Mehta, R.G.; et al. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science 1997, 275, 218–220. [Google Scholar] [CrossRef]
- Athar, M.; Back, J.H.; Tang, X.; Kim, K.H.; Kopelovich, L.; Bickers, D.R.; Kim, A.L. Resveratrol: A review of preclinical studies for human cancer prevention. Toxicol. Appl. Phamacol. 2007, 224, 274–283. [Google Scholar] [CrossRef]
- Delmas, D.; Lancon, A.; Colin, D.; Jannin, B.; Latruffe, N. Resveratrol as a chemopreventive agent: A promising molecule for fighting cancer. Curr. Drug Targets 2006, 7, 423–442. [Google Scholar] [CrossRef]
- Sharma, S.; Anjaneyulu, M.; Kulkarni, S.K.; Chopra, K. Resveratrol, a polyphenolic phytoalexin, attenuates diabetic nephropathy in rats. Pharmacology 2006, 76, 69–75. [Google Scholar] [CrossRef]
- Su, H.C.; Hung, L.M.; Chen, J.K. Resveratrol, a red wine antioxidant, possesses an insulin-like effect in streptozotocin-induced diabetic rats. Am. J. Physiol. Endocrinol. Metab. 2006, 290, E1339–E1346. [Google Scholar] [CrossRef]
- Szkudelski, T.; Szkudelska, K. Anti-diabetic effects of resveratrol. Ann. N. Y. Acad. Sci. 2011, 1215, 34–39. [Google Scholar] [CrossRef]
- De La Lastra, C.A.; Villegas, I. Resveratrol as an anti-inflammatory and anti-aging agent: Mechanisms and clinical implications. Mol. Nutr. Food Res. 2005, 49, 405–430. [Google Scholar] [CrossRef]
- Lee, B.; Moon, S.K. Resveratrol inhibits TNF-alpha-induced proliferation and matrix metalloproteinase expression in human vascular smooth muscles. J. Nutr. 2005, 135, 2767–2773. [Google Scholar]
- Martin, A.R.; Villegas, I.; Sanchez-Hidalgo, M.; de la Lastra, C.A. The effects of resveratrol, a phytoalexin derived from red wines, on chronic inflammation induced in an experimentally induced colitis model. Br. J. Pharmacol. 2006, 147, 873–885. [Google Scholar] [CrossRef]
- Rahman, I.; Marwick, J.; Kirkham, P. Redox modulation of chromatin remodeling: Impact on histone acetylation and deacetylation, NF-kappaB and proinflammatory gene expression. Biochem. Pharmacol. 2004, 68, 1255–1267. [Google Scholar] [CrossRef]
- Valenzano, D.R.; Terzibasi, E.; Genade, T.; Cattaneo, A.; Domenici, L.; Cellerino, A. Resveratrol prolongs lifespan and retards the onset of age-related markers in a short-lived vertebrate. Curr. Biol. 2006, 16, 296–300. [Google Scholar] [CrossRef]
- Valenzano, D.R.; Cellerino, A. Resveratrol and the pharmacology of aging: A new vertebrate model to validate an old molecule. Cell Cycle 2006, 5, 1027–1032. [Google Scholar] [CrossRef]
- Kaga, S.; Zhan, L.; Matsumoto, M.; Maulik, N. Resveratrol enhances neovascularization in the infarcted rat myocardium through the induction of thioredoxin-1, heme oxygenase-1 and vascular endothelial growth factor. J. Mol. Cell. Cardiol. 2005, 39, 813–822. [Google Scholar] [CrossRef]
- Khan, A.A.; Dace, D.S.; Ryazanov, A.G.; Kelly, J.; Apte, R.S. Resveratrol regulates pathologic angiogenesis by a eukaryotic elongation factor-2 kinase-regulated pathway. Am. J. Pathol. 2010, 177, 481–492. [Google Scholar] [CrossRef]
- Chen, Y.; Tseng, S.H. Review. Pro- and anti-angiogenesis effects of resveratrol. In Vivo 2007, 21, 365–370. [Google Scholar]
- Robich, M.P.; Osipov, R.M.; Nezafat, R.; Feng, J.; Clements, R.T.; Bianchi, C.; Boodhwani, M.; Coady, M.A.; Laham, R.J.; Sellke, F.W. Resveratrol improves myocardial perfusion in a swine model of hypercholesterolemia and chronic myocardial ischemia. Circulation 2010, 122, S142–S149. [Google Scholar] [CrossRef]
- Srivastava, R.K.; Unterman, T.G.; Shankar, S. FOXO transcription factors and VEGF neutralizing antibody enhance antiangiogenic effects of resveratrol. Mol. Cell. Biochem. 2010, 337, 201–212. [Google Scholar] [CrossRef]
- Kimura, Y.; Sumiyoshi, M.; Baba, K. Antitumor activities of synthetic and natural stilbenes through antiangiogenic action. Cancer Sci. 2008, 99, 2083–2096. [Google Scholar] [CrossRef]
- Garvin, S.; Ollinger, K.; Dabrosin, C. Resveratrol induces apoptosis and inhibits angiogenesis in human breast cancer xenografts in vivo. Cancer Lett. 2006, 231, 113–122. [Google Scholar] [CrossRef]
- Chen, J.C.; Chen, Y.; Lin, J.H.; Wu, J.M.; Tseng, SH. Resveratrol suppresses angiogenesis in gliomas: Evaluation by color Doppler ultrasound. Anticancer Res. 2006, 26, 1237–1245. [Google Scholar]
- Bagchi, D.; Garg, A.; Krohn, R.L.; Bagchi, M.; Tran, M.X.; Stohs, S.J. Oxygen free radical scavenging abilities of vitamins C and E, and a grape seed proanthocyanidin extract in vitro. Res. Commun. Mol. Pathol. Pharmacol. 1997, 95, 179–189. [Google Scholar]
- Bagchi, D.; Bagchi, M.; Stohs, S.J.; Ray, S.D.; Sen, C.K.; Preuss, H.G. Cellular protection with proanthocyanidins derived from grape seeds. Ann. N. Y. Acad. Sci. 2002, 957, 260–270. [Google Scholar] [CrossRef]
- Terra, X.; Montagut, G.; Bustos, M.; Llopiz, N.; Ardèvol, A.; Bladé, C.; Fernández-Larrea, J.; Pujadas, G.; Salvadó, J.; Arola, L.; et al. Grape-seed procyanidins prevent low-grade inflammation by modulating cytokine expression in rats fed a high-fat diet. J. Nutr. Biochem. 2009, 20, 210–218. [Google Scholar] [CrossRef]
- Ye, X.; Krohn, R.L.; Liu, W.; Joshi, S.S.; Kuszynski, C.A.; McGinn, T.R.; Bagchi, M.; Preuss, H.G.; Stohs, S.J.; Bagchi, D. The cytotoxic effects of a novel IH636 grape seed proanthocyanidin extract on cultured human cancer cells. Mol. Cell. Biochem. 1999, 196, 99–108. [Google Scholar] [CrossRef]
- Pataki, T.; Bak, I.; Kovacs, P.; Baqchi, D.; Das, D.K.; Tosaki, A. Grape seed proanthocyanidins improved cardiac recovery during reperfusion after ischemia in isolated rat hearts. Am. J. Clin. Nutr. 2002, 75, 894–899. [Google Scholar]
- Bagchi, D.; Garg, A.; Krohn, R.L.; Bagchi, M.; Bagchi, D.J.; Stohs, S. Protective effects of grape seed proanthocyanidins and selected antioxidants against TPA-induced hepatic and brain lipid peroxidation and DNA fragmentation, and peritoneal macrophage activation in mice. Gen. Pharm. 1998, 30, 771–776. [Google Scholar] [CrossRef]
- Song, X.; Xu, H.; Feng, Y.; Li, X.; Lin, M.; Cao, L. Protective effect of grape seed proanthocyanidins against liver ischemic reperfusion injury: Particularly in diet-induced obese mice. Int. J. Biol. Sci. 2012, 8, 1345–1362. [Google Scholar] [CrossRef]
- Khanna, S.; Roy, S.; Bagchi, D.; Bagchi, M.; Sen, C.K. Upregulation of oxidant-induced VEGF expression in cultured keratinocytes by a grape seed proanthocyanidin extract. Free Radic. Biol. Med. 2001, 31, 38–42. [Google Scholar] [CrossRef]
- Khanna, S.; Venojarvi, M.; Roy, S.; Sharma, N.; Trikha, P.; Bagchi, D.; Bagchi, M.; Sen, C.K. Dermal wound healing properties of redox-active grape seed proanthocyanidins. Free Radic. Biol. Med. 2002, 33, 1089–1096. [Google Scholar] [CrossRef]
- Pesca, M.S.; Dal Piaz, F.; Sanogo, R.; Vassallo, A.; Bruzual de Abreu, M.; Rapisarda, A.; Germanò, M.P.; Certo, G.; de falco, S.; de tommasi, N.; et al. Bioassay-guided isolation of proanthocyanidins with antiangiogenic activities. J. Nat. Prod. 2013, 76, 29–35. [Google Scholar] [CrossRef]
- Huang, S.; Yang, N.; Liu, Y.; Hu, L.; Zhao, J.; Gao, J.; Li, Y.; Li, C.; Zhang, X.; Huang, T. Grape seed proanthocyanidins inhibit angiogenesis via the downregulation of both vascular endothelial growth factor and angiopoietin signaling. Nutr. Res. 2012, 32, 530–536. [Google Scholar] [CrossRef]
- Li, W.; Jiang, D. Effect of resveratrol on bcl-2 and VEGF expression in oxygen-induced retinopathy of prematurity. J. Pediatr. Ophthalmol. Strabismus 2012, 49, 230–235. [Google Scholar] [CrossRef]
- Hua, J.; Guerin, K.I.; Chen, J.; Michán, S.; Stahl, A.; Krah, N.M.; Seaward, M.R.; Dennison, R.J.; Juan, A.M.; Hatton, C.J.; et al. Resveratrol inhibits pathologic retinal neovascularization in Vldlr(-/-) mice. Investig. Ophthalmol. Vis. Sci. 2011, 52, 2809–2816. [Google Scholar] [CrossRef]
- Kim, Y.H.; Kim, Y.S.; Roh, G.S.; Choi, W.S.; Cho, G.J. Resveratrol blocks diabetes-induced early vascular lesions and vascular endothelial growth factor induction in mouse retinas. Acta Ophthalmol. 2012, 90, 31–37. [Google Scholar] [CrossRef]
- Sareen, D.; van Ginkel, P.R.; Takach, J.C.; Mohiuddin, A.; Darjatmoko, S.R.; Albert, D.M.; Polans, A.S. Mitochondria are the primary target during resveratrol-induced apoptosis in human retinoblastoma cells. Investig. Ophthalmol. Vis. Sci. 2006, 47, 3708–3716. [Google Scholar] [CrossRef]
- Sareen, D.; Darjatmoko, S.R.; Albert, D.M.; Polans, A.S. Mitochondria, calcium, and calpain are key mediators of resveratrol-induced apoptosis in breast cancer. Mol. Pharm. 2007, 72, 1466–1475. [Google Scholar] [CrossRef]
- Van Ginkel, P.R.; Sareen, D.; Subramanian, L.; Walker, Q.; Darjatmoko, S.R.; Lindstrom, M.; Kulkarni, A.; Albert, D.M.; Polans, A.S. Resveratrol inhibits tumor growth of human neuroblastoma and mediates apoptosis by directly targeting mitochondria. Clin. Cancer Res. 2007, 13, 5162–5169. [Google Scholar] [CrossRef]
- Teodoro, J.G.; Evans, S.K.; Green, M.R. Inhibition of tumor angiogenesis by p53: A new role for the guardian of the genome. J. Mol. Med. 2007, 85, 1175–1186. [Google Scholar] [CrossRef]
- Busquets, S.; Ametller, E.; Fuster, G.; Olivan, M.; Raab, V.; Argilés, J.M.; Lopez-Soriano, F.J. Resveratrol, a natural diphenol, reduces metastatic growth in an experimental cancer model. Cancer Lett. 2007, 245, 144–148. [Google Scholar] [CrossRef]
- Bhattacharya, S.S.; Darjatmoko, S.R.; Polans, A.S. The inactivation of Akt/PKB by resveratrol regulates the malignant properties of cutaneous melanoma. Melanoma Res. 2011, 21, 180–187. [Google Scholar] [CrossRef]
- Karar, J.; Maity, A. PI3K/AKT/mTOR pathway in angiogenesis. Front. Mol. Neurosci. 2011, 4, 51–65. [Google Scholar] [CrossRef]
- Berridge, M.J.; Lipp, P.; Bootman, M.D. The versatility and universality of calcium signaling. Nat. Rev. Mol. Cell Biol. 2000, 1, 11–21. [Google Scholar] [CrossRef]
- Campos-Toimil, M.; Elies, J.; Alvarez, E.; Verde, I.; Orallo, F. Effects of trans- and cis-resveratrol on Ca2+ handling in A7r5 vascular myocytes. Eur. J. Pharmacol. 2007, 577, 91–99. [Google Scholar] [CrossRef]
- Ma, X.; Tian, X.; Huang, X.; Yan, F.; Qiao, D. Resveratrol-induced mitochondrial dysfunction and apoptosis are associated with calcium and mCICR-mediated MPT activation in HepG2 cells. Mol. Cell. Biochem. 2007, 302, 99–109. [Google Scholar] [CrossRef]
- Miki, K.; Miki, A.; Matsuoka, M.; Muramatsu, D.; Hackett, S.F.; Campochiaro, P.A. Effects of intraocular ranibizumab and bevacizumab in transgenic mice expressing human vascular endothelial growth factor. Ophthalmology 2009, 116, 1748–1754. [Google Scholar] [CrossRef]
- Walle, T. Bioavailability of resveratrol. Ann. N. Y. Acad. Sci. 2011, 1215, 9–15. [Google Scholar] [CrossRef]
- Walle, T.; Hsieh, F.; DeLegge, M.H.; Oatis, J.E., Jr.; Walle, U.K. High absorption but very low bioavailability of oral resveratrol in humans. Drug Metab. Dispos. 2004, 32, 1377–1382. [Google Scholar] [CrossRef]
- Wenzel, E.; Somoza, V. Metabolism and bioavailability of trans-resveratrol. Mol. Nutr. Food Res. 2005, 49, 472–481. [Google Scholar] [CrossRef]
- Gescher, A.J.; Steward, W.P. Relationship between mechanisms, bioavailability, and preclinical chemopreventive efficacy of resveratrol: A conundrum. Cancer Epidemiol. Biomark. Prev. 2003, 12, 953–957. [Google Scholar]
- Asensi, M.; Medina, I.; Ortega, A.; Carretero, J.; Baño, M.C.; Obrador, E.; Estrela, J.M. Inhibition of cancer growth by resveratrol is related to its low bioavailability. Free Radic. Biol. Med. 2002, 33, 387–398. [Google Scholar] [CrossRef]
- Robich, M.P.; Chu, L.M.; Chaudray, M.; Nezafat, R.; Han, Y.; Clements, R.T.; Laham, R.J.; Manning, W.J.; Coady, M.A.; Sellke, F.W. Anti-angiogenic effect of high-dose resveratrol in a swine model of metabolic syndrome. Surgery 2010, 148, 453–462. [Google Scholar] [CrossRef]
- Tseng, S.-H.; Lin, S.-M.; Chen, J.-C.; Su, Y.H.; Huang, H.Y.; Chen, C.K.; Lin, P.Y.; Chen, Y. Resveratrol suppresses the angiogenesis and tumor growth of gliomas in rats. Clin. Cancer Res. 2004, 10, 2190–2202. [Google Scholar] [CrossRef]
- Dudley, J.; Das, S.; Mukherjee, S.; Das, D.K. Resveratrol, a unique phytoalexin present in red wine, delivers either survival signal or death signal to the ischemic myocardium depending on dose. J. Nutr. Biochem. 2009, 20, 443–452. [Google Scholar] [CrossRef]
- Nagineni, C.; Raju, R.; Nagineni, K.; Kommineni, V.; Cherukuri, A.; Kutty, R.; Hooks, J.; Detrick, B. Resveratrol suppresses expression of VEGF by human retinal pigment epithelial cells: Potential nutraceutical for age-related macular degeneration. Aging Dis. 2014, 5, 88–100. [Google Scholar]
- Stewart, E.; Samaranayake, G.; Browning, A.; Hopkinson, A.; Amoaku, W. Comparison of choroidal and retinal endothelial cells: Characteristics and response to VEGF isoforms and anti-VEGF treatments. Exp. Eye Res. 2011, 93, 761–766. [Google Scholar] [CrossRef]
- Nagai, N.; Kubota, S.; Tsubota, K.; Ozawa, Y. Resveratrol prevents the development of choroidal neovascularization by modulating AMP-activated protein kinase in macrophages and other cell types. J. Nutr. Biochem. 2014, 25, 1218–1225. [Google Scholar] [CrossRef]
- Richer, S.; Stiles, W.; Ulanski, L.; Carroll, D.; Podella, C. Observation of human retinal remodeling in octogenarians with a resveratrol based nutritional supplement. Nutrients 2013, 5, 1989–2005. [Google Scholar] [CrossRef]
- Maltepe, E.; Schmidt, J.; Baunoch, D.; Bradfield, C.; Simon, M. Abnormal angiogenesis and responses to glucose and oxygen deprivation in mice lacking the protein ARNT. Nature 1997, 386, 403–407. [Google Scholar] [CrossRef]
- Wen, W.; Lu, J.; Zhang, K.; Chen, S. Grape seed extract inhibits angiogenesis via suppression of the vascular endothelial growth factor receptor signaling pathway. Cancer Prev. Res. 2008, 1, 554–561. [Google Scholar] [CrossRef]
- Chen, Y.; Li, X.; Xing, N.; Cao, X. Quercetin inhibits choroidal and retinal angiogenesis in vitro. Graefe’s Arch. Clin. Exp. Ophthalmol. 2008, 246, 373–378. [Google Scholar] [CrossRef]
- Zhuang, P.; Shen, Y.; Lin, B.; Zhang, W.; Chiou, G. Effect of quercetin on formation of choroidal neovascularization (CNV) in age-related macular degeneration (AMD). Eye Sci. 2011, 26, 23–29. [Google Scholar]
- Donnini, S.; Finetti, F.; Lusini, L.; Morbidelli, L.; Cheynier, V.; Barron, D.; Williamson, G.; Waltenberger, J.; Ziche, M. Divergent effects of quercetin conjugates on angiogenesis. Br. J. Nutr. 2006, 95, 1016–1023. [Google Scholar] [CrossRef]
- Lavine, J.A.; Sang, Y.; Wang, S.; Ip, M.S.; Sheibani, N. Attenuation of choroidal neovascularization by β2-adrenoreceptor antagonism. JAMA Ophthalmol. 2013, 131, 376–382. [Google Scholar] [CrossRef]
- Wang, S.; Sorenson, C.M.; Sheibani, N. Lack of thrombospondin 1 and exacerbation of choroidal neovascularization. Arch. Ophthalmol. 2012, 130, 615–620. [Google Scholar]
- Grynkiewicz, G.; Poenie, M.; Tsien, R.Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J. Biol. Chem. 1985, 260, 3440–3450. [Google Scholar]
- Sample Availability: Compounds are commercially available.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kanavi, M.R.; Darjatmoko, S.; Wang, S.; Azari, A.A.; Farnoodian, M.; Kenealey, J.D.; Van Ginkel, P.R.; Albert, D.M.; Sheibani, N.; Polans, A.S. The Sustained Delivery of Resveratrol or a Defined Grape Powder Inhibits New Blood Vessel Formation in a Mouse Model of Choroidal Neovascularization. Molecules 2014, 19, 17578-17603. https://doi.org/10.3390/molecules191117578
Kanavi MR, Darjatmoko S, Wang S, Azari AA, Farnoodian M, Kenealey JD, Van Ginkel PR, Albert DM, Sheibani N, Polans AS. The Sustained Delivery of Resveratrol or a Defined Grape Powder Inhibits New Blood Vessel Formation in a Mouse Model of Choroidal Neovascularization. Molecules. 2014; 19(11):17578-17603. https://doi.org/10.3390/molecules191117578
Chicago/Turabian StyleKanavi, Mozhgan Rezaie, Soesiawati Darjatmoko, Shoujian Wang, Amir A. Azari, Mitra Farnoodian, Jason D. Kenealey, Paul R. Van Ginkel, Daniel M. Albert, Nader Sheibani, and Arthur S. Polans. 2014. "The Sustained Delivery of Resveratrol or a Defined Grape Powder Inhibits New Blood Vessel Formation in a Mouse Model of Choroidal Neovascularization" Molecules 19, no. 11: 17578-17603. https://doi.org/10.3390/molecules191117578