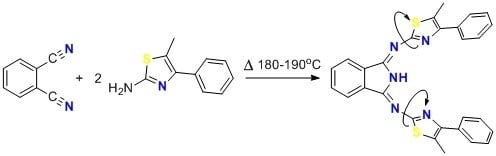
1,3-Bis(5′-methyl-4′-phenyl-2′-thiazolylimino)isoindoline
Abstract
:1. Introduction
2. Results and Discussion

3. Experimental Section
3.1. General Methods, Analytical and Physical Measurements
3.2. Synthesis of 1,3-Bis(5′-methyl-4′-phenyl-2′-thiazolylimino)isoindoline (1)

Supplementary Materials
Supplementary File 1Supplementary File 2Supplementary File 3Supplementary File 4Acknowledgments
Author Contributions
Conflicts of Interest
References
- Csonka, R.; Speier, G.; Kaizer, J. Isoindoline-derived ligands and applications. RSC Adv. 2015, 5, 18401–18419. [Google Scholar] [CrossRef]
- Bröring, M.; Kleeberg, C. Facile intramolecular C(sp3)–H bond activation with PdII. Chem. Commun. 2008, 2777–2778. [Google Scholar] [CrossRef] [PubMed]
- Bröring, M.; Kleeberg, C. Cyclometalation vs. Werner-type coordination of sterically enforced palladium(II)-1,3-bis(pyridyl-2-imino)isoindolines (Pd-BPIs). Dalton Trans. 2007, 1101–1103. [Google Scholar] [CrossRef] [PubMed]
- Pap, J.S.; Cranswick, M.A.; Balogh-Hergovich, É.; Baráth, G.; Giorgi, M.; Rohde, G.T.; Kaizer, J.; Speier, G.; Que, L., Jr. An Iron(II)[1,3-bis(2′-pyridylimino)isoindoline] Complex as a Catalyst for Substrate Oxidation with H2O2—Evidence for a Transient Peroxidodiiron(III) Species. Eur. J. Inorg. Chem. 2013, 3858–3866. [Google Scholar] [CrossRef] [PubMed]
- Siegl, W.O. A new bis-cheiating ligand system. Synthesis and chelating behavior. Inorg. Chim. Acta 1977, 25, L65–L66. [Google Scholar] [CrossRef]
- Elvidge, J.A.; Linstead, R.P. Heterocyclic Imines. Part I. Imino-derivatives of isoindoline and their reaction with primary bases. J. Chem. Soc. 1952, 5000–5007. [Google Scholar] [CrossRef]
- Domaille, P.J.; Harlow, R.L.; Ittel, S.D.; Peet, W.G. NMR spectra of paramagnetic group 8 complexes of bis(pyridy1imino)isoindoline. Inorg. Chem. 1983, 22, 3944–3952. [Google Scholar] [CrossRef]
- Siegl, W.O. Metal Ion Activation of Nitriles. Syntheses of 1,3-bis( arylimino)isoindolines. J. Org. Chem. 1977, 42, 1872–1878. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Csonka, R.; Szávuly, M.I.; Giorgi, M.; Speier, G.; Kaizer, J. 1,3-Bis(5′-methyl-4′-phenyl-2′-thiazolylimino)isoindoline. Molbank 2016, 2016, M882. https://doi.org/10.3390/M882
Csonka R, Szávuly MI, Giorgi M, Speier G, Kaizer J. 1,3-Bis(5′-methyl-4′-phenyl-2′-thiazolylimino)isoindoline. Molbank. 2016; 2016(1):M882. https://doi.org/10.3390/M882
Chicago/Turabian StyleCsonka, Róbert, Miklós István Szávuly, Michel Giorgi, Gábor Speier, and József Kaizer. 2016. "1,3-Bis(5′-methyl-4′-phenyl-2′-thiazolylimino)isoindoline" Molbank 2016, no. 1: M882. https://doi.org/10.3390/M882