Gene Expression of Haloferax volcanii on Intermediate and Abundant Sources of Fixed Nitrogen
Abstract
:1. Introduction
2. Results and Discussion
2.1. Genome-Wide Expression Analysis under Different Nitrogen Sources
2.2. Putative Amino Acid Transport Systems were Upregulated on l-Alanine
2.3. Upregulation of Metabolic Systems
2.4. Identification of a Candidate Regulator and cis-Sequence for Coordinated Transcriptional Control
3. Materials and Methods
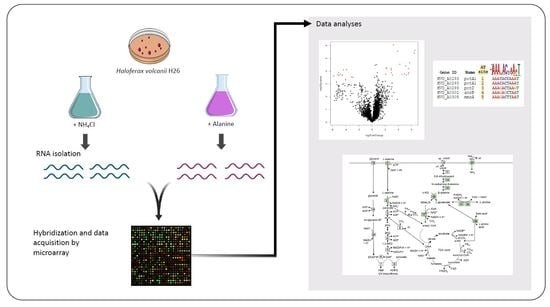
3.1. Growth of H. volcanii
3.2. Preparation and Analysis of Microarray Data
3.3. Computational Prediction of Transcription Factor Binding Sequences
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A. Supplemental Figures



Appendix B. Supplemental Methods
Appendix B.1. Growth Assay
Appendix B.2. Quantitative PCR to Calculate Copy Number and Gene Expression
| Name | Sequence (5′-3′) | Description | Ref. |
|---|---|---|---|
| HVO0863external_FW | TCCCGGAGAGGTGTGTATGT | To test DNA contamination. Targeting up/downstream of HVO_0863. Amplicon: 1,767 bp. | This study |
| HVO0863external_RV | TCACTGACCTCGAACACGTC | To test DNA contamination. Targeting up/downstream of HVO_0863. Amplicon: 1,767 bp. | This study |
| rpl16s1_F | CCACGTCATCCGCGAGAACA | Control locus in main chromosome. Amplicon: 86 bp. | [66] |
| rpl16s1_R | CGACCTTCCCGAACGACTGG | Control locus in main chromosome. Amplicon: 86 bp. | [66] |
| qHVO_C0033_s2_F | CTCGGTTCGACAGGCCAAGG | Target locus in pHV1. Amplicon: 113 bp. | This study |
| qHVO_C0033_s2_R | CAGTTCCGCACATGGCATCG | Target locus in pHV1. Amplicon: 113 bp. | This study |
| qHVO_C0069_s2_F | AGGCACCGACTGTCTCGTCA | Target locus in pHV1. Amplicon: 124 bp. | This study |
| qHVO_C0069_s2_R | GAAGGAGAATCGCGCGGTGA | Target locus in pHV1. Amplicon: 124 bp. | This study |
| qHVO_A0299_s2_F | ACCGCCTACGGAACCTCAGT | Target locus in pHV4. Amplicon: 126 bp. | This study |
| qHVO_A0299_s2_R | CTCGGACATCGTGGCGTAGG | Target locus in pHV4. Amplicon: 126 bp. | This study |
| qHVO_0454_s2_F | CGGAGCTCATCTCGGCCATC | Target locus in main chromosome. Amplicon: 92 bp. | This study |
| qHVO_0454_s2_R | CTGCGGCAGGTCGATGTAGG | Target locus in main chromosome. Amplicon: 92 bp. | This study |
| qHVO_0086_s1_F | CGCGTGGATTGACTGGCTCT | Target locus in main chromosome. Amplicon: 100 bp. | This study |
| qHVO_0086_s1_R | GATGTACGCGCGGAAGTCCA | Target locus in main chromosome. Amplicon: 100 bp. | This study |
| qHVO_1189_s1_F | CTCCGAGGACTACCGCCAGA | Target locus in main chromosome. Amplicon: 81 bp. | This study |
| qHVO_1189_s1_R | CCGATACACGGGAGGTTGCC | Target locus in main chromosome. Amplicon: 81 bp. | This study |
| qHVO_2307_s1_F | TTCGACCTCGACGGAACCCT | Target locus in main chromosome. Amplicon: 127 bp. | This study |
| qHVO_2307_s1_R | CGAGTCCGTGCCAGATGGTC | Target locus in main chromosome. Amplicon: 127 bp. | This study |
| qHVO_C0064_s1_F | GCGAATACGTCGTCCACGGT | Target locus in pHV1. Amplicon: 70 bp. | This study |
| qHVO_C0064_s1_R | TGGCTCTCGCAGCTGTTGAC | Target locus in pHV1. Amplicon: 70 bp. | This study |
References
- Alwine, J.C.; Kemp, D.J.; Stark, G.R. Method for detection of specific RNAs in agarose gels by transfer to diazobenzyloxymethyl-paper and hybridization with DNA probes. Proc. Natl. Acad. Sci. USA 1977, 74, 5350–5354. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.M.; Doyle, M.V.; Mark, D.F. Quantitation of mRNA by the polymerase chain reaction. Proc. Natl. Acad. Sci. USA 1989, 86, 9717–9721. [Google Scholar] [CrossRef] [PubMed]
- Selinger, D.W.; Cheung, K.J.; Mei, R.; Johansson, E.M.; Richmond, C.S.; Blattner, F.R.; Lockhart, D.J.; Church, G.M. RNA expression analysis using a 30 base pair resolution Escherichia coli genome array. Nat. Biotechnol. 2000, 18, 1262–1268. [Google Scholar] [CrossRef] [PubMed]
- Lashkari, D.A.; DeRisi, J.L.; McCusker, J.H.; Namath, A.F.; Gentile, C.; Hwang, S.Y.; Brown, P.O.; Davis, R.W. Yeast microarrays for genome wide parallel genetic and gene expression analysis. Proc. Natl. Acad. Sci. USA 1997, 94, 13057–13062. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagalakshmi, U.; Wang, Z.; Waern, K.; Shou, C.; Raha, D.; Gerstein, M.; Snyder, M. The transcriptional landscape of the yeast genome defined by RNA sequencing. Science 2008, 320, 1344–1349. [Google Scholar] [CrossRef] [PubMed]
- Mortazavi, A.; Williams, B.A.; McCue, K.; Schaeffer, L.; Wold, B. Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat. Methods 2008, 5, 621–628. [Google Scholar] [CrossRef]
- Danan, M.; Schwartz, S.; Edelheit, S.; Sorek, R. Transcriptome-wide discovery of circular RNAs in Archaea. Nucleic Acids Res. 2012, 40, 3131–3142. [Google Scholar] [CrossRef] [PubMed]
- Esclapez, J.; Pire, C.; Camacho, M.; Bautista, V.; Martinez-Espinosa, R.M.; Zafrilla, B.; Vegara, A.; Alcaraz, L.A.; Bonete, M.J. Transcriptional profiles of Haloferax mediterranei based on nitrogen availability. J. Biotechnol. 2015, 193, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Twellmeyer, J.; Wende, A.; Wolfertz, J.; Pfeiffer, F.; Panhuysen, M.; Zaigler, A.; Soppa, J.; Welzl, G.; Oesterhelt, D. Microarray analysis in the archaeon Halobacterium salinarum strain R1. PLoS ONE 2007, 2, e1064. [Google Scholar] [CrossRef]
- Schmid, A.K.; Reiss, D.J.; Pan, M.; Koide, T.; Baliga, N.S. A single transcription factor regulates evolutionarily diverse but functionally linked metabolic pathways in response to nutrient availability. Mol. Syst. Biol. 2009, 5, 282. [Google Scholar] [CrossRef]
- Richardson, D.J.; Watmough, N.J. Inorganic nitrogen metabolism in bacteria. Curr. Opin. Chem. Biol. 1999, 3, 207–219. [Google Scholar] [CrossRef]
- Stahl, D.A.; de la Torre, J.R. Physiology and diversity of ammonia-oxidizing archaea. Annu. Rev. Microbiol. 2012, 66, 83–101. [Google Scholar] [CrossRef]
- Kowalchuk, G.A.; Stephen, J.R. Ammonia-oxidizing bacteria: A model for molecular microbial ecology. Annu. Rev. Microbiol. 2001, 55, 485–529. [Google Scholar] [CrossRef]
- van Spanning, R.J.M.; Richardson, D.J.; Ferguson, S.J. Introduction to the Biochemistry and Molecular Biology of Denitrification; Elsevier B.V.: Amsterdam, The Netherlands, 2007. [Google Scholar]
- Bae, H.S.; Morrison, E.; Chanton, J.P.; Ogram, A. Methanogens are major contributors to nitrogen fixation in soils of the Florida Everglades. Appl. Environ. Microbiol. 2018, 84, e02222-17. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, B.M.; Lukoyanov, D.; Yang, Z.Y.; Dean, D.R.; Seefeldt, L.C. Mechanism of nitrogen fixation by nitrogenase: The next stage. Chem. Rev. 2014, 114, 4041–4062. [Google Scholar] [CrossRef]
- Lam, P.; Kuypers, M.M. Microbial nitrogen cycling processes in oxygen minimum zones. Ann. Rev. Mar. Sci. 2011, 3, 317–345. [Google Scholar] [CrossRef] [PubMed]
- Ward, M.H.; Jones, R.R.; Brender, J.D.; de Kok, T.M.; Weyer, P.J.; Nolan, B.T.; Villanueva, C.M.; van Breda, S.G. Drinking water nitrate and human health: An updated review. Int. J. Environ. Res. Public Health 2018, 15, 1557. [Google Scholar] [CrossRef]
- Griffis, T.J.; Chen, Z.; Baker, J.M.; Wood, J.D.; Millet, D.B.; Lee, X.; Venterea, R.T.; Turner, P.A. Nitrous oxide emissions are enhanced in a warmer and wetter world. Proc. Natl. Acad. Sci. USA 2017, 114, 12081–12085. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vassart, A.; Van Wolferen, M.; Orell, A.; Hong, Y.; Peeters, E.; Albers, S.V.; Charlier, D. Sa-Lrp from Sulfolobus acidocaldarius is a versatile, glutamine-responsive, and architectural transcriptional regulator. Microbiologyopen 2013, 2, 75–93. [Google Scholar] [CrossRef]
- Jager, D.; Sharma, C.M.; Thomsen, J.; Ehlers, C.; Vogel, J.; Schmitz, R.A. Deep sequencing analysis of the Methanosarcina mazei Go1 transcriptome in response to nitrogen availability. Proc. Natl. Acad. Sci. USA 2009, 106, 21878–21882. [Google Scholar] [CrossRef]
- Prasse, D.; Schmitz, R.A. Small RNAs involved in regulation of nitrogen metabolism. Microbiol. Spectr. 2018, 6. [Google Scholar] [CrossRef]
- Esclapez, J.; Bravo-Barrales, G.; Bautista, V.; Pire, C.; Camacho, M.; Bonete, M.J. Effects of nitrogen sources on the nitrate assimilation in Haloferax mediterranei: Growth kinetics and transcriptomic analysis. FEMS Microbiol. Lett. 2014, 350, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Paya, G.; Bautista, V.; Camacho, M.; Castejon-Fernandez, N.; Alcaraz, L.A.; Bonete, M.J.; Esclapez, J. Small RNAs of Haloferax mediterranei: Identification and potential involvement in nitrogen metabolism. Genes 2018, 9, 83. [Google Scholar] [CrossRef] [PubMed]
- Mancinelli, R.L.; Hochstein, L.I. The occurrence of denitrification in extremely halophilic bacteria. FEMS Microbiol. Lett. 1986, 35, 55–58. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Espinosa, R.M.; Marhuenda-Egea, F.C.; Bonete, M.J. Purification and characterisation of a possible assimilatory nitrite reductase from the halophile archaeon Haloferax mediterranei. FEMS Microbiol. Lett. 2001, 196, 113–118. [Google Scholar] [CrossRef]
- Pedro-Roig, L.; Lange, C.; Bonete, M.J.; Soppa, J.; Maupin-Furlow, J. Nitrogen regulation of protein-protein interactions and transcript levels of GlnK PII regulator and AmtB ammonium transporter homologs in Archaea. MicrobiologyOpen 2013, 2, 826–840. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mullakhanbhai, M.F.; Larsen, H. Halobacterium volcanii spec. nov., a Dead Sea halobacterium with a moderate salt requirement. Arch. Microbiol. 1975, 104, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Stiller, M.; Nissenbaum, A. Geochemical investigation of phosphorus and nitrogen in the hypersaline Dead Sea. Geochim. Cosmochim. Acta 1999, 63, 3467–3475. [Google Scholar] [CrossRef]
- Large, A.; Stamme, C.; Lange, C.; Duan, Z.; Allers, T.; Soppa, J.; Lund, P.A. Characterization of a tightly controlled promoter of the halophilic archaeon Haloferax volcanii and its use in the analysis of the essential cct1 gene. Mol. Microbiol. 2007, 66, 1092–1106. [Google Scholar] [CrossRef] [PubMed]
- Lie, T.J.; Leigh, J.A. Regulatory response of Methanococcus maripaludis to alanine, an intermediate nitrogen source. J. Bacteriol. 2002, 184, 5301–5306. [Google Scholar] [CrossRef]
- Huerta-Cepas, J.; Szklarczyk, D.; Forslund, K.; Cook, H.; Heller, D.; Walter, M.C.; Rattei, T.; Mende, D.R.; Sunagawa, S.; Kuhn, M.; et al. eggNOG 4.5: A hierarchical orthology framework with improved functional annotations for eukaryotic, prokaryotic and viral sequences. Nucleic Acids Res. 2016, 44, D286–D293. [Google Scholar] [CrossRef] [PubMed]
- Dulmage, K.A.; Darnell, C.L.; Vreugdenhil, A.; Schmid, A.K. Copy number variation is associated with gene expression change in archaea. Microb. Genom. 2018, 4, e000210. [Google Scholar] [CrossRef]
- Maurer, S.; Ludt, K.; Soppa, J. Characterization of copy number control of two Haloferax volcanii replication origins using deletion mutants and haloarchaeal artificial chromosomes. J. Bacteriol. 2018, 200, e00517-17. [Google Scholar] [CrossRef] [PubMed]
- Ausiannikava, D.; Mitchell, L.; Marriott, H.; Smith, V.; Hawkins, M.; Makarova, K.S.; Koonin, E.V.; Nieduszynski, C.A.; Allers, T. Evolution of genome architecture in archaea: Spontaneous generation of a new chromosome in Haloferax volcanii. Mol. Biol. Evol. 2018, 35, 1855–1868. [Google Scholar] [CrossRef] [PubMed]
- Zerulla, K.; Baumann, A.; Soppa, J. Cell Cycle and Polyploidy in Haloarchaea; Caister Academic Press: Wymondham, UK, 2014; pp. 145–166. [Google Scholar]
- Conroy, M.J.; Durand, A.; Lupo, D.; Li, X.D.; Bullough, P.A.; Winkler, F.K.; Merrick, M. The crystal structure of the Escherichia coli AmtB-GlnK complex reveals how GlnK regulates the ammonia channel. Proc. Natl. Acad. Sci. USA 2007, 104, 1213–1218. [Google Scholar] [CrossRef] [PubMed]
- Kadaba, N.S.; Kaiser, J.T.; Johnson, E.; Lee, A.; Rees, D.C. The high-affinity E. coli methionine ABC transporter: Structure and allosteric regulation. Science 2008, 321, 250–253. [Google Scholar] [CrossRef] [PubMed]
- Schroder, I.; Vadas, A.; Johnson, E.; Lim, S.; Monbouquette, H.G. A novel archaeal alanine dehydrogenase homologous to ornithine cyclodeaminase and mu-crystallin. J. Bacteriol. 2004, 186, 7680–7689. [Google Scholar] [CrossRef] [PubMed]
- Walker, M.C.; van der Donk, W.A. The many roles of glutamate in metabolism. J. Ind. Microbiol. Biotechnol. 2016, 43, 419–430. [Google Scholar] [CrossRef]
- Shi, L.; Tu, B.P. Acetyl-CoA and the regulation of metabolism: Mechanisms and consequences. Curr. Opin. Cell Biol. 2015, 33, 125–131. [Google Scholar] [CrossRef]
- Tzeng, C.T.; Huang, Y.H.; Huang, C.Y. Crystal structure of dihydropyrimidinase from Pseudomonas aeruginosa PAO1: Insights into the molecular basis of formation of a dimer. Biochem. Biophys. Res. Commun. 2016, 478, 1449–1455. [Google Scholar] [CrossRef]
- Humble, M.S.; Cassimjee, K.E.; Hakansson, M.; Kimbung, Y.R.; Walse, B.; Abedi, V.; Federsel, H.J.; Berglund, P.; Logan, D.T. Crystal structures of the Chromobacterium violaceum ω-transaminase reveal major structural rearrangements upon binding of coenzyme PLP. FEBS J. 2012, 279, 779–792. [Google Scholar] [CrossRef] [PubMed]
- Stines-Chaumeil, C.; Talfournier, F.; Branlant, G. Mechanistic characterization of the MSDH (methylmalonate semialdehyde dehydrogenase) from Bacillus subtilis. Biochem. J. 2006, 395, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Allers, T.; Ngo, H.P.; Mevarech, M.; Lloyd, R.G. Development of additional selectable markers for the halophilic archaeon Haloferax volcanii based on the leuB and trpA genes. Appl. Environ. Microbiol. 2004, 70, 943–953. [Google Scholar] [CrossRef] [PubMed]
- Johnsen, U.; Reinhardt, A.; Landan, G.; Tria, F.D.K.; Turner, J.M.; Davies, C.; Schonheit, P. New views on an old enzyme: Allosteric regulation and evolution of archaeal pyruvate kinases. FEBS J. 2019, 286, 2471–2489. [Google Scholar] [CrossRef] [PubMed]
- Pickl, A.; Johnsen, U.; Schönheit, P. Fructose degradation in the haloarchaeon Haloferax volcanii involves a bacterial type phosphoenolpyruvate-dependent phosphotransferase system, fructose-1-phosphate kinase, and class II fructose-1,6-bisphosphate aldolase. J. Bacteriol. 2012, 194, 3088–3097. [Google Scholar] [CrossRef] [PubMed]
- Walden, H.; Taylor, G.; Lilie, H.; Knura, T.; Hensel, R. Triosephosphate isomerase of the hyperthermophile Thermoproteus tenax: Thermostability is not everything. Biochem. Soc. Trans. 2004, 32, 305. [Google Scholar] [CrossRef] [PubMed]
- Sutter, J.M.; Tastensen, J.B.; Johnsen, U.; Soppa, J.; Schonheit, P. Key enzymes of the semiphosphorylative Entner-Doudoroff pathway in the haloarchaeon Haloferax volcanii: Characterization of glucose dehydrogenase, gluconate dehydratase, and 2-keto-3-deoxy-6-phosphogluconate aldolase. J. Bacteriol. 2016, 198, 2251–2262. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, H.; Izui, K.; Mizuguchi, K. A novel mechanism of allosteric regulation of archaeal phosphoenolpyruvate carboxylase: A combined approach to structure-based alignment and model assessment. Protein Eng. Des. Sel. 2006, 19, 409–419. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, R.; Satomura, T.; Sakuraba, H.; Ohshima, T. L-proline dehydrogenases in hyperthermophilic archaea: Distribution, function, structure, and application. Appl. Microbiol. Biotechnol. 2012, 93, 83–93. [Google Scholar] [CrossRef]
- Singh, H.; Arentson, B.W.; Becker, D.F.; Tanner, J.J. Structures of the PutA peripheral membrane flavoenzyme reveal a dynamic substrate-channeling tunnel and the quinone-binding site. Proc. Natl. Acad. Sci. USA 2014, 111, 3389–3394. [Google Scholar] [CrossRef] [Green Version]
- Botou, M.; Lazou, P.; Papakostas, K.; Lambrinidis, G.; Evangelidis, T.; Mikros, E.; Frillingos, S. Insight on specificity of uracil permeases of the NAT/NCS2 family from analysis of the transporter encoded in the pyrimidine utilization operon of Escherichia coli. Mol. Microbiol. 2018, 108, 204–219. [Google Scholar] [CrossRef] [PubMed]
- Rawls, K.S.; Martin, J.H.; Maupin-Furlow, J.A. Activity and transcriptional regulation of bacterial protein-like glycerol-3-phosphate dehydrogenase of the haloarchaea in Haloferax volcanii. J. Bacteriol. 2011, 193, 4469–4476. [Google Scholar] [CrossRef] [PubMed]
- Sherwood, K.E.; Cano, D.J.; Maupin-Furlow, J.A. Glycerol-mediated repression of glucose metabolism and glycerol kinase as the sole route of glycerol catabolism in the haloarchaeon Haloferax volcanii. J. Bacteriol. 2009, 191, 4307–4315. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Orell, A.; Maes, D.; van Wolferen, M.; Lindas, A.C.; Bernander, R.; Albers, S.V.; Charlier, D.; Peeters, E. BarR, an Lrp-type transcription factor in Sulfolobus acidocaldarius, regulates an aminotransferase gene in a beta-alanine responsive manner. Mol. Microbiol. 2014, 92, 625–639. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, K.; Lindås, A.C.; Peeters, E. The genome-scale DNA-binding profile of BarR, a β-alanine responsive transcription factor in the archaeon Sulfolobus acidocaldarius. BMC Genom. 2016, 17, 569. [Google Scholar] [CrossRef] [PubMed]
- Okamura, H.; Yokoyama, K.; Koike, H.; Yamada, M.; Shimowasa, A.; Kabasawa, M.; Kawashima, T.; Suzuki, M. A structural code for discriminating between transcription signals revealed by the feast/famine regulatory protein DM1 in complex with ligands. Structure 2007, 15, 1325–1338. [Google Scholar] [CrossRef] [PubMed]
- Dyall-Smith, M. The Halohandbook. 2019. Available online: https://haloarchaea.com/wp-content/uploads/2018/10/Halohandbook_2009_v7.3mds.pdf (accessed on 26 September 2019).
- Darnell, C.L.; Tonner, P.D.; Gulli, J.G.; Schmidler, S.C.; Schmid, A.K. Systematic discovery of archaeal transcription factor functions in regulatory networks through quantitative phenotyping analysis. MSystems 2017, 2, e00032-17. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.H.; Sherwood Rawls, K.; Chan, J.C.; Hwang, S.; Martinez-Pastor, M.; McMillan, L.J.; Prunetti, L.; Schmid, A.K.; Maupin-Furlow, J.A. GlpR is a direct transcriptional repressor of fructose metabolic genes in Haloferax volcanii. J. Bacteriol. 2018, 200, e00244-18. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Bailey, T.L.; Boden, M.; Buske, F.A.; Frith, M.; Grant, C.E.; Clementi, L.; Ren, J.; Li, W.W.; Noble, W.S. MEME SUITE: Tools for motif discovery and searching. Nucleic Acids Res. 2009, 37, W202–W208. [Google Scholar] [CrossRef]
- Jiang, M.; Anderson, J.; Gillespie, J.; Mayne, M. uShuffle: A useful tool for shuffling biological sequences while preserving the k-let counts. BMC Bioinform. 2008, 9, 192. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Liu, R.; Sanchez, I.; Silva-Sanchez, C.; Hepowit, N.L.; Cao, S.; Chen, S.; Maupin-Furlow, J. Ubiquitin-like proteasome system represents a eukaryotic-like pathway for targeted proteolysis in archaea. MBio 2016, 7, e00379-16. [Google Scholar] [CrossRef] [PubMed]






| Uniprot Annotation 1; Proposed Function 2 | Gene | Log2 Fold Change | SD 3 | SD Error |
|---|---|---|---|---|
| ABC-type transport system periplasmic substrate-binding protein (probable substrate spermidine/putrescine); l-alanine transport | HVO_A0299, potD 5 | 6.495 | 0.177 | 0.102 |
| Polysaccharide deacetylase family protein | HVO_A0301, nodB-like | 6.416 | 0.280 | 0.161 |
| (Methyl)malonate-semialdehyde dehydrogenase [EC:1.2.1.27] | HVO_A0305, mmsA 5 | 5.701 | 0.310 | 0.179 |
| Luciferase family protein; oxidoreductase | HVO_A0295A | 5.569 | 0.396 | 0.228 |
| ABC-type transport system permease protein (probable substrate spermidine/putrescine); l-alanine transport | HVO_A0300, potB 4 | 5.530 | 0.609 | 0.351 |
| Amidase (hydantoinase/carbamoylase family); β-ureidopropionase / N-carbamoyl-l-amino-acid hydrolase [EC:3.5.1.6/3.5.1.87] | HVO_A0295, amaB2 4 | 5.504 | 0.637 | 0.368 |
| Putative allantoinase; dihydropyrimidinase [EC 3.5.2.2] | HVO_A0303, dpyS 5 | 5.200 | 0.771 | 0.445 |
| ABC-type transport system ATP-binding protein (probable substrate spermidine/putrescine); l-alanine transport | HVO_A0294, potA2 4 | 5.119 | 0.293 | 0.169 |
| ABC-type transport system ATP-binding protein (probable substrate spermidine/putrescine); l-alanine transport | HVO_A0293, potA1 4,5 | 4.913 | 0.656 | 0.379 |
| Pyridoxal phosphate-dependent aminotransferase | HVO_A0306, gabT6 4,5 | 4.555 | 0.508 | 0.293 |
| Nitrogen regulatory protein P-II | HVO_0085, glnK2 4 | 4.203 | 0.241 | 0.139 |
| Transport protein (Probable substrate ammonium) | HVO_0086, amt2 | 3.898 | 0.165 | 0.095 |
| Short-chain family oxidoreductase | HVO_A0296 | 3.244 | 0.106 | 0.061 |
| Alanine dehydrogenase [EC 1.4.1.1] | HVO_0454, ala 4 | 3.107 | 0.537 | 0.31 |
| Nitrogen regulatory protein P-II | HVO_0083, glnK1 4,5 | 2.922 | 0.303 | 0.175 |
| Proline dehydrogenase [EC:1.5.5.2] | HVO_1191, fadM2 4 | 2.901 | 0.280 | 0.161 |
| Uncharacterized protein (arCOG09242) | HVO_0210 | 2.898 | 0.335 | 0.193 |
| HAD superfamily hydrolase with phosphoglycolate phosphatase-like, domain 2 (IPR023198) | HVO_2307 | 2.22 | 0.270 | 0.156 |
| Uncharacterized protein of arCOG06214 | HVO_2678 | 2.113 | 0.231 | 0.133 |
| Aldehyde dehydrogenase; delta-1-pyrroline-5-carboxylate dehydrogenase [EC:1.2.1.3] | HVO_1189, aldH2 | 2.086 | 0.243 | 0.140 |
| Glucose-fructose oxidoreductase | HVO_C0069, gfo | −2.949 | 0.167 | 0.096 |
| HTH domain protein | HVO_C0051 5 | −3.156 | 0.414 | 0.239 |
| Uncharacterized protein | HVO_C0010 | −3.918 | 0.376 | 0.217 |
| Uncharacterized protein | HVO_C0033 | −4.347 | 0.541 | 0.313 |
| Small CPxCG-related zinc finger protein | HVO_C0086 | −4.522 | 0.381 | 0.220 |
| UPF0395 family protein | HVO_C0006 | −4.792 | 0.715 | 0.413 |
| Homolog to virus protein eHPD7_00180 | HVO_C0085 | −5.018 | 0.585 | 0.338 |
| ISH4-type transposase ISHvo5 | HVO_C0064 | −5.027 | 0.662 | 0.382 |
| hypothetical protein | HVO_C0019 | −5.227 | 0.778 | 0.449 |
| hypothetical protein | HVO_C0088 | −5.779 | 0.837 | 0.483 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hwang, S.; Chavarria, N.E.; Hackley, R.K.; Schmid, A.K.; Maupin-Furlow, J.A. Gene Expression of Haloferax volcanii on Intermediate and Abundant Sources of Fixed Nitrogen. Int. J. Mol. Sci. 2019, 20, 4784. https://doi.org/10.3390/ijms20194784
Hwang S, Chavarria NE, Hackley RK, Schmid AK, Maupin-Furlow JA. Gene Expression of Haloferax volcanii on Intermediate and Abundant Sources of Fixed Nitrogen. International Journal of Molecular Sciences. 2019; 20(19):4784. https://doi.org/10.3390/ijms20194784
Chicago/Turabian StyleHwang, Sungmin, Nikita E. Chavarria, Rylee K. Hackley, Amy K. Schmid, and Julie A. Maupin-Furlow. 2019. "Gene Expression of Haloferax volcanii on Intermediate and Abundant Sources of Fixed Nitrogen" International Journal of Molecular Sciences 20, no. 19: 4784. https://doi.org/10.3390/ijms20194784