Use of Curcumin, a Natural Polyphenol for Targeting Molecular Pathways in Treating Age-Related Neurodegenerative Diseases
Abstract
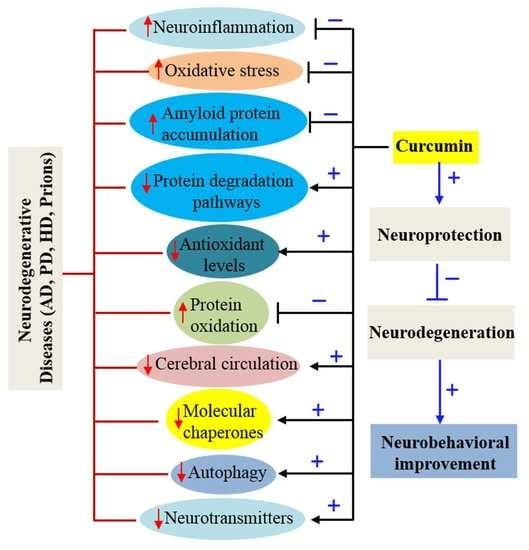
:1. Introduction
2. Curcumin: The Major Active Polyphenol of Turmeric
2.1. Source
2.2. Chemistry of Cur
2.3. Cur Biosynthesis
2.4. Cur Metabolism
3. Pleotropic Actions of Cur on Nervous System
3.1. Anti-Amyloid Properties
3.2. Potent Antioxidant
3.3. Anti-Inflammatory Agent
3.4. Modulate Activity of Molecular Chaperones
3.5. Increase Neurotrophins, Neurogenesis and Synaptogenesis
3.6. Metal Chelator
3.7. Cur Regulates Epigenetics
3.8. Improving Cerebral Circulation
4. Limitations of Cur Delivery
5. Nano-Technological Approaches for Cur Delivery
5.1. Adjuvants
5.2. Bio-Conjugates
5.3. Cur-Phospholipid Complex
5.4. Liposomes
5.5. Micelles
5.6. Noisome
5.7. Nanogels
5.8. Chitosan
5.9. Gold Particles
5.10. Silver Particles
5.11. Cyclodextrin
5.12. Dendrimer
5.13. Solid Lipid Nanoparticles (SLNP)
5.14. Derivatives and Analogues of Cur
6. Rationale for Cur Therapy in Neurodegenerative Diseases
6.1. Curcumin Therapy in Alzheimer’s Disease
6.1.1. Inhibition of Aβ Aggregation
6.1.2. Inhibition of Aβ Production
6.1.3. Aβ Clearance
6.1.4. Inhibition of Tau Phosphorylation
6.1.5. Inhibition of Oxidation and Inflammation
6.1.6. As an Imaging Probe for Aβ-Plaque Detection Ex Vivo and In Vivo
6.2. Curcumin Therapy in Parkinson’s Disease
Effects of Cur on PD
6.3. Curcumin in Huntington’s Disease
Beneficial effects of Cur in Huntington Disease
6.4. Curcumin Therapy in Prion Diseases
6.5. Effects of Cur on Prion Disease
7. Biphasic or Dose-Dependent Effects of Curcumin
8. Recommended Doses and Limitations of Cur Therapy
9. Future Perspective of Curcumin Research
10. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
Abbreviations
| Cur | Curcumin |
| CNS | Central nervous system |
| AD | Alzheimer disease |
| SLN | Solid lipid nanoparticles |
| DMC | Demethoxycurucmin |
| BDMC | Bisdemethoxycurucmin |
| IUPAC | International Union of Pure and Applied Chemistry |
| DMSO | Dimethyl-sulfoxide |
| DMF | Dimethyl formamide |
| PK | Pharmacokinetics |
| PD | Pharmacodynamics |
| THC | Tetrahydrocurcumin |
| HHC | Hexahydrocurcumin |
| i.v. | Intravenously |
| i.p. | Intraperitonally |
| OHC | Octahydrocurcumin |
| Aβ | Amyloid β-protein |
| α-syn | Alfa-synuclein |
| HTT | Huntingtin |
| PD | Parkinson’s disease |
| HD | Huntington’s disease |
| p-tau | Phosphorylated tau |
| BBB | Blood brain barrier |
| PUFA | Polyunsaturated fatty acids |
| ROS | Reactive oxygen species |
| GSH | Glutathione (reduced) |
| SOD | Superoxide dismutase |
| GPx | Glutathione peroxidase |
| GST | Glutathione S-transferase |
| NF-κB | Nuclear factor κ-light-chain-enhancer of activated B cells transcription factor |
| COX | Cyclooxygenase |
| LOX | Lipoxygenase |
| TNF | Tumor necrosis factor |
| IL | Interleukin |
| PPARγ | Peroxisome proliferator-activated receptor gamma |
| iNOS | Induced nitric oxide synthase |
| HSP | Heat shock protein |
| NGF | Nerve growth factor |
| BDNF | Brain derived neurotropic factor |
| GDNF | Glial derived neurotropic factor |
| PDGF | Platelet derived neurotropic factor |
| PSD | Post-synaptic density protein |
| HAT | Histone acetyltransferase |
| BMECs | Brain microvascular endothelial cells |
| GI | Gastrointestinal |
| EGCG | Epigallocatechin gallate |
| GMO | Genetically modified organism |
| PLGA | Poly lactic-co-glycolic acid |
| BCM-95 | Biocurcumax-95 |
| IC | Inhibitory concentration |
| DLS | Dynamic light scattering |
| SEM | Scanning electron microscope |
| FTIR | Fourier transforms infrared spectroscopy |
| ER | Endoplasmic reticulum |
| AuNPs | Gold nanoparticles |
| AgNPs | Silver nanocomposite |
| Cds | Cyclodextrins |
| SLNP | Solid lipid nanoparticles |
| nm | Nanometer |
| nM | Nanomolar |
| ppm | Parts-per-million |
| SLCP | Solid lipid Cur particle |
| NFT | Neurofibrillary tangle |
| BACE | β-secretase |
| APP | Amyloid precursor protein |
| PHF | Pair helical filaments |
| GSK-3β | Glycogen synthase kinase-3β |
| MAPK | Mitogen-activated protein kinase |
| Cdk5 | Cyclin-dependent kinase 5 |
| ERK2 | Extracellular signal-regulated kinase 2 |
| MARK | Microtubule affinity-regulating kinase |
| SADK | SAD-kinase |
| PKA | Protein kinase A |
| CaMKII | Calcium/calmodulin-dependent protein kinase II |
| JNKc | Jun N-terminal kinase |
| IRS | Insulin receptor substrate |
| GFAP | Glial fibrillary acidic protein |
| Iba-1 | Ionized calcium-binding adapter molecule 1 |
| PET | Positron emission tomography |
| NIR | Near infrared |
| PIB | Pittsburgh compound B |
| FDG | 2-Deoxy-2-[18F] fluoroglucose |
| SNpc | Substantia nigra pars compacta |
| DA | Dopamine |
| LBs | Lewy bodies |
| 2D-NMR | Two-dimensional nuclear magnetic resonance |
| MAO | Monoamine oxidase |
| PolyQ | Poly-glutamine |
| mHTT | Mutated huntingtin protein |
| CAG | Cytosine-adenine-guanine |
| YAC | Yeast artificial chromosome |
| DARPP | Dopamine-and cAMP-regulated neuronal phosphoprotein |
| TSE | Transmissible spongiform encephalopathies |
| PrP | Prion protein |
| PrPC | Cellular form of prion protein |
| NSAIDs | Non-steroidal anti-inflammatory drugs |
| NPI-Q | Neuropsychiatric Inventory–Questionnaire |
| PBS | Phosphate Buffer Saline |
References
- Kaytor, M.D.; Warren, S.T. Aberrant protein deposition and neurological disease. J. Biol. Chem. 1999, 274, 37507–37510. [Google Scholar] [CrossRef] [PubMed]
- Selkoe, D.J. Cell biology of protein misfolding: The examples of Alzheimer’s and Parkinson’s diseases. Nat. Cell Biol. 2004, 6, 1054–1061. [Google Scholar] [CrossRef] [PubMed]
- Amor, S.; Puentes, F.; Baker, D.; van der Valk, P. Inflammation in neurodegenerative diseases. Immunology 2010, 129, 154–169. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Guo, C.; Kong, J. Oxidative stress in neurodegenerative diseases. Neural Regen. Res. 2012, 7, 376–385. [Google Scholar] [PubMed]
- Cummings, J.; Lee, G.; Mortsdorf, T.; Ritter, A.; Zhong, K. Alzheimer’s disease drug development pipeline: 2017. Alzheimer’s Dement. 2017, 3, 367–384. [Google Scholar] [CrossRef] [PubMed]
- Koronyo, Y.; Salumbides, B.C.; Black, K.L.; Koronyo-Hamaoui, M. Alzheimer’s disease in the retina: Imaging retinal abeta plaques for early diagnosis and therapy assessment. Neurodegener. Dis. 2012, 10, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Palanivelu, K. The effect of curcumin (turmeric) on Alzheimer’s disease: An overview. Ann. Indian Acad. Neurol. 2008, 11, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Prasad, S.; Aggarwal, B.B. Turmeric, the Golden Spice: From Traditional Medicine. In Herbal Medicine: Biomolecular and Clinical Aspects, 2nd ed.; Benzie, I.F.F., Wachtel-Galor, S., Eds.; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar]
- Yang, F.; Lim, G.P.; Begum, A.N.; Ubeda, O.J.; Simmons, M.R.; Ambegaokar, S.S.; Chen, P.P.; Kayed, R.; Glabe, C.G.; Frautschy, S.A.; et al. Curcumin inhibits formation of amyloid beta oligomers and fibrils, binds plaques, and reduces amyloid in vivo. J. Biol. Chem. 2005, 280, 5892–5901. [Google Scholar] [CrossRef] [PubMed]
- Begum, A.N.; Jones, M.R.; Lim, G.P.; Morihara, T.; Kim, P.; Heath, D.D.; Rock, C.L.; Pruitt, M.A.; Yang, F.; Hudspeth, B.; et al. Curcumin structure-function, bioavailability, and efficacy in models of neuroinflammation and Alzheimer’s disease. J. Pharmacol. Exp. Ther. 2008, 326, 196–208. [Google Scholar] [CrossRef] [PubMed]
- Ghalandarlaki, N.; Alizadeh, A.M.; Ashkani-Esfahani, S. Nanotechnology-applied curcumin for different diseases therapy. Biomed. Res. Int. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.L.; Zuo, X.; Yang, F.; Ubeda, O.J.; Gant, D.J.; Alaverdyan, M.; Teng, E.; Hu, S.; Chen, P.P.; Maiti, P.; et al. Curcumin suppresses soluble tau dimers and corrects molecular chaperone, synaptic, and behavioral deficits in aged human tau transgenic mice. J. Biol. Chem. 2013, 288, 4056–4065. [Google Scholar] [CrossRef] [PubMed]
- Maiti, P.; Hall, T.C.; Paladugu, L.; Kolli, N.; Learman, C.; Rossignol, J.; Dunbar, G.L. A comparative study of dietary curcumin, nanocurcumin, and other classical amyloid-binding dyes for labeling and imaging of amyloid plaques in brain tissue of 5x-familial Alzheimer’s disease mice. Histochem. Cell Biol. 2016, 146, 609–625. [Google Scholar] [CrossRef] [PubMed]
- Maiti, P.; Al-Gharaibeh, A.; Kolli, N.; Dunbar, G.L. Solid Lipid Curcumin Particles Induce More DNA Fragmentation and Cell Death in Cultured Human Glioblastoma Cells than Does Natural Curcumin. Oxid. Med. Cell. Longev. 2017, 2017. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.H.; Loo, C.Y.; Bebawy, M.; Luk, F.; Mason, R.S.; Rohanizadeh, R. Curcumin and its derivatives: Their application in neuropharmacology and neuroscience in the 21st century. Curr. Neuropharmacol. 2013, 11, 338–378. [Google Scholar] [CrossRef] [PubMed]
- Priyadarsini, K.I. Chemical and structural features influencing the biological activity of curcumin. Curr. Pharm. Des. 2013, 19, 2093–2100. [Google Scholar] [PubMed]
- Galer, P.; Golobic, A.; Koller, J.; Kosmrlj, B.; Sket, B. Structures in solid state and solution of dimethoxy curcuminoids: Regioselective bromination and chlorination. Chem. Cent. J. 2013, 7, 107. [Google Scholar] [CrossRef] [PubMed]
- Amalraj, A.; Pius, A.; Gopi, S.; Gopi, S. Biological activities of curcuminoids, other biomolecules from turmeric and their derivatives—A review. J. Tradit. Complement. Med. 2017, 7, 205–233. [Google Scholar] [CrossRef] [PubMed]
- Priyadarsini, K.I. The chemistry of curcumin: From extraction to therapeutic agent. Molecules 2014, 19, 20091–20112. [Google Scholar] [CrossRef] [PubMed]
- Katsuyama, Y.; Kita, T.; Funa, N.; Horinouchi, S. Curcuminoid biosynthesis by two type III polyketide synthases in the herb Curcuma longa. J. Biol. Chem. 2009, 284, 11160–11170. [Google Scholar] [CrossRef] [PubMed]
- Katsuyama, Y.; Kita, T.; Horinouchi, S. Identification and characterization of multiple curcumin synthases from the herb Curcuma longa. FEBS Lett. 2009, 583, 2799–2803. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.A.; McLelland, H.R.; Hill, K.A.; Ireson, C.R.; Euden, S.A.; Manson, M.M.; Pirmohamed, M.; Marnett, L.J.; Gescher, A.J.; Steward, W.P. Pharmacodynamic and pharmacokinetic study of oral Curcuma extract in patients with colorectal cancer. Clin. Cancer Res. 2001, 7, 1894–1900. [Google Scholar] [PubMed]
- Heger, M.; van Golen, R.F.; Broekgaarden, M.; Michel, M.C. The molecular basis for the pharmacokinetics and pharmacodynamics of curcumin and its metabolites in relation to cancer. Pharmacol. Rev. 2014, 66, 222–307. [Google Scholar] [CrossRef] [PubMed]
- DiSilvestro, R.A.; Joseph, E.; Zhao, S.; Bomser, J. Diverse effects of a low dose supplement of lipidated curcumin in healthy middle aged people. Nutr. J. 2012, 11, 79. [Google Scholar] [CrossRef] [PubMed]
- Koronyo-Hamaoui, M.; Koronyo, Y.; Ljubimov, A.V.; Miller, C.A.; Ko, M.K.; Black, K.L.; Schwartz, M.; Farkas, D.L. Identification of amyloid plaques in retinas from Alzheimer’s patients and noninvasive in vivo optical imaging of retinal plaques in a mouse model. Neuroimage 2011, 54, S204–S217. [Google Scholar] [CrossRef] [PubMed]
- Anand, P.; Kunnumakkara, A.B.; Newman, R.A.; Aggarwal, B.B. Bioavailability of curcumin: Problems and promises. Mol. Pharm. 2007, 4, 807–818. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.H.; Huang, T.M.; Lin, J.K. Biotransformation of curcumin through reduction and glucuronidation in mice. Drug Metab. Dispos. 1999, 27, 486–494. [Google Scholar] [PubMed]
- Vareed, S.K.; Kakarala, M.; Ruffin, M.T.; Crowell, J.A.; Normolle, D.P.; Djuric, Z.; Brenner, D.E. Pharmacokinetics of curcumin conjugate metabolites in healthy human subjects. Cancer Epidemiol. Biomarkers Prev. 2008, 17, 1411–1417. [Google Scholar] [CrossRef] [PubMed]
- Jager, R.; Lowery, R.P.; Calvanese, A.V.; Joy, J.M.; Purpura, M.; Wilson, J.M. Comparative absorption of curcumin formulations. Nutr. J. 2014, 13, 11. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Bhattacharyya, S.; Rashid, K.; Sil, P.C. Curcumin protects rat liver from streptozotocin-induced diabetic pathophysiology by counteracting reactive oxygen species and inhibiting the activation of p53 and MAPKs mediated stress response pathways. Toxicol. Rep. 2015, 2, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Sandur, S.K.; Pandey, M.K.; Sung, B.; Ahn, K.S.; Murakami, A.; Sethi, G.; Limtrakul, P.; Badmaev, V.; Aggarwal, B.B. Curcumin, demethoxycurcumin, bisdemethoxycurcumin, tetrahydrocurcumin and turmerones differentially regulate anti-inflammatory and anti-proliferative responses through a ROS-independent mechanism. Carcinogenesis 2007, 28, 1765–1773. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.B.; Sundaram, C.; Malani, N.; Ichikawa, H. Curcumin: The Indian solid gold. Adv. Exp. Med. Biol. 2007, 595, 1–75. [Google Scholar] [PubMed]
- Cole, G.M.; Teter, B.; Frautschy, S.A. Neuroprotective effects of curcumin. Adv. Exp. Med. Biol. 2007, 595, 197–212. [Google Scholar] [PubMed]
- Yanagisawa, D.; Taguchi, H.; Yamamoto, A.; Shirai, N.; Hirao, K.; Tooyama, I. Urcuminoid binds to amyloid-beta1-42 oligomer and fibril. J. Alzheimer’s Dis. 2011, 24, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.K.; Kotia, V.; Ghosh, D.; Mohite, G.M.; Kumar, A.; Maji, S.K. Curcumin modulates alpha-synuclein aggregation and toxicity. ACS Chem. Neurosci. 2013, 4, 393–407. [Google Scholar] [CrossRef] [PubMed]
- Chongtham, A.; Agrawal, N. Curcumin modulates cell death and is protective in Huntington’s disease model. Sci. Rep. 2016, 6, 18736. [Google Scholar] [CrossRef] [PubMed]
- Mohorko, N.; Repovs, G.; Popovic, M.; Kovacs, G.G.; Bresjanac, M. Curcumin labeling of neuronal fibrillar tau inclusions in human brain samples. J. Neuropathol. Exp. Neurol. 2010, 69, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Hafner-Bratkovic, I.; Gaspersic, J.; Smid, L.M.; Bresjanac, M.; Jerala, R. Curcumin binds to the alpha-helical intermediate and to the amyloid form of prion protein—A new mechanism for the inhibition of PrP(Sc) accumulation. J. Neurochem. 2008, 104, 1553–1564. [Google Scholar] [CrossRef] [PubMed]
- Mythri, R.B.; Bharath, M.M. Curcumin: A potential neuroprotective agent in Parkinson’s disease. Curr. Pharm. Des. 2012, 18, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Hickey, M.A.; Zhu, C.; Medvedeva, V.; Lerner, R.P.; Patassini, S.; Franich, N.R.; Maiti, P.; Frautschy, S.A.; Zeitlin, S.; Levine, M.S.; et al. Improvement of neuropathology and transcriptional deficits in CAG 140 knock-in mice supports a beneficial effect of dietary curcumin in Huntington’s disease. Mol. Neurodegener. 2012, 7, 12. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.F.; Yu, K.H.; Jheng, C.P.; Chung, R.; Lee, C.I. Curcumin reduces amyloid fibrillation of prion protein and decreases reactive oxidative stress. Pathogens 2013, 2, 506–519. [Google Scholar] [CrossRef] [PubMed]
- Mosley, R.L.; Benner, E.J.; Kadiu, I.; Thomas, M.; Boska, M.D.; Hasan, K.; Laurie, C.; Gendelman, H.E. Neuroinflammation, Oxidative Stress and the Pathogenesis of Parkinson’s Disease. Clin. Neurosci. Res. 2006, 6, 261–281. [Google Scholar] [CrossRef] [PubMed]
- Gregersen, N.; Bross, P. Protein misfolding and cellular stress: An overview. Methods Mol. Biol. 2010, 648, 3–23. [Google Scholar] [PubMed]
- Menon, V.P.; Sudheer, A.R. Antioxidant and anti-inflammatory properties of curcumin. Adv. Exp. Med. Biol. 2007, 595, 105–125. [Google Scholar] [PubMed]
- Biswas, S.K.; McClure, D.; Jimenez, L.A.; Megson, I.L.; Rahman, I. Curcumin induces glutathione biosynthesis and inhibits NF-kappaB activation and interleukin-8 release in alveolar epithelial cells: Mechanism of free radical scavenging activity. Antioxid. Redox Signal. 2005, 7, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Jat, D.; Parihar, P.; Kothari, S.C.; Parihar, M.S. Curcumin reduces oxidative damage by increasing reduced glutathione and preventing membrane permeability transition in isolated brain mitochondria. Cell Mol. Biol. (Noisy-le-grand) 2013, 59, 1899–1905. [Google Scholar]
- Jones, D.P. Radical-free biology of oxidative stress. Am. J. Physiol. Cell Physiol. 2008, 295, C849–C868. [Google Scholar] [CrossRef] [PubMed]
- Sies, H. Oxidative stress: A concept in redox biology and medicine. Redox Biol. 2015, 4, 180–183. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Yue, Y.; Zheng, X.; Zhang, K.; Chen, S.; Du, Z. Curcumin, inflammation, and chronic diseases: How are they linked? Molecules 2015, 20, 9183–9213. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Bose, M.; Ju, J.; Ryu, J.H.; Chen, X.; Sang, S.; Lee, M.J.; Yang, C.S. Modulation of arachidonic acid metabolism by curcumin and related beta-diketone derivatives: Effects on cytosolic phospholipase A(2), cyclooxygenases and 5-lipoxygenase. Carcinogenesis 2004, 25, 1671–1679. [Google Scholar] [CrossRef] [PubMed]
- Lim, G.P.; Chu, T.; Yang, F.; Beech, W.; Frautschy, S.A.; Cole, G.M. The curry spice curcumin reduces oxidative damage and amyloid pathology in an Alzheimer transgenic mouse. J. Neurosci. 2001, 21, 8370–8377. [Google Scholar] [CrossRef] [PubMed]
- Gulcubuk, A.; Altunatmaz, K.; Sonmez, K.; Haktanir-Yatkin, D.; Uzun, H.; Gurel, A.; Aydin, S. Effects of curcumin on tumour necrosis factor-alpha and interleukin-6 in the late phase of experimental acute pancreatitis. J. Vet. Med. A Physiol. Pathol. Clin. Med. 2006, 53, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Mazidi, M.; Karimi, E.; Meydani, M.; Ghayour-Mobarhan, M.; Ferns, G.A. Potential effects of curcumin on peroxisome proliferator-activated receptor-gamma in vitro and in vivo. World J. Methodol. 2016, 6, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Leak, R.K. Heat shock proteins in neurodegenerative disorders and aging. J. Cell Commun. Signal. 2014, 8, 293–310. [Google Scholar] [CrossRef] [PubMed]
- Maiti, P.; Dunbar, G.L. Comparative Neuroprotective Effects of Dietary Curcumin and Solid Lipid Curcumin Particles in Cultured Mouse Neuroblastoma Cells after Exposure to Abeta42. Int. J. Alzheimer’s Dis. 2017, 2017. [Google Scholar] [CrossRef]
- Gupta, S.C.; Prasad, S.; Kim, J.H.; Patchva, S.; Webb, L.J.; Priyadarsini, I.K.; Aggarwal, B.B. Multitargeting by curcumin as revealed by molecular interaction studies. Nat. Prod. Rep. 2011, 28, 1937–1955. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Zeng, Q.; Mitchell, E.S.; Xiu, J.; Duan, Y.; Li, C.; Tiwari, J.K.; Hu, Y.; Cao, X.; Zhao, Z. Curcumin enhances neurogenesis and cognition in aged rats: Implications for transcriptional interactions related to growth and synaptic plasticity. PLoS ONE 2012, 7, e31211. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, T.; Enam, S.A.; Gilani, A.H. Curcuminoids enhance memory in an amyloid-infused rat model of Alzheimer’s disease. Neuroscience 2010, 169, 1296–1306. [Google Scholar] [CrossRef] [PubMed]
- Perry, G.; Sayre, L.M.; Atwood, C.S.; Castellani, R.; Cash, A.D.; Rottkamp, C.A.; Smith, M.A. The role of iron and copper in the aetiology of neurodegenerative disorders: Therapeutic implications. CNS Drugs 2002, 16, 339–352. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Huang, W.; Moir, R.D.; Vanderburg, C.R.; Lai, B.; Peng, Z.; Tanzi, R.E.; Rogers, J.T.; Huang, X. Metal exposure and Alzheimer’s pathogenesis. J. Struct. Biol. 2006, 155, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Wanninger, S.; Lorenz, V.; Subhan, A.; Edelmann, F.T. Metal complexes of curcumin—Synthetic strategies, structures and medicinal applications. Chem. Soc. Rev. 2015, 44, 4986–5002. [Google Scholar] [CrossRef] [PubMed]
- Eybl, V.; Kotyzova, D.; Bludovska, M. The effect of curcumin on cadmium-induced oxidative damage and trace elements level in the liver of rats and mice. Toxicol. Lett. 2004, 151, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Shakibaei, M.; John, T.; Schulze-Tanzil, G.; Lehmann, I.; Mobasheri, A. Suppression of NF-kappaB activation by curcumin leads to inhibition of expression of cyclo-oxygenase-2 and matrix metalloproteinase-9 in human articular chondrocytes: Implications for the treatment of osteoarthritis. Biochem. Pharmacol. 2007, 73, 1434–1445. [Google Scholar] [CrossRef] [PubMed]
- Reuter, S.; Gupta, S.C.; Park, B.; Goel, A.; Aggarwal, B.B. Epigenetic changes induced by curcumin and other natural compounds. Genes Nutr. 2011, 6, 93–108. [Google Scholar] [CrossRef] [PubMed]
- Rose, N.R.; Klose, R.J. Understanding the relationship between DNA methylation and histone lysine methylation. Biochim. Biophys. Acta 2014, 1839, 1362–1372. [Google Scholar] [CrossRef] [PubMed]
- Awad, A.S. Effect of combined treatment with curcumin and candesartan on ischemic brain damage in mice. J. Stroke Cerebrovasc. Dis. 2011, 20, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Gu, Z.L.; Qin, Z.H.; Liang, Z.Q. Effect of curcumin on the adhesion of platelets to brain microvascular endothelial cells in vitro. Acta Pharmacol. Sin. 2008, 29, 800–807. [Google Scholar] [CrossRef] [PubMed]
- Vachharajani, V.; Wang, S.W.; Mishra, N.; El Gazzar, M.; Yoza, B.; McCall, C. Curcumin modulates leukocyte and platelet adhesion in murine sepsis. Microcirculation 2010, 17, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Avci, G.; Kadioglu, H.; Sehirli, A.O.; Bozkurt, S.; Guclu, O.; Arslan, E.; Muratli, S.K. Curcumin protects against ischemia/reperfusion injury in rat skeletal muscle. J. Surg. Res. 2012, 172, e39–e46. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.C.; Patchva, S.; Aggarwal, B.B. Therapeutic roles of curcumin: Lessons learned from clinical trials. AAPS J. 2013, 15, 195–218. [Google Scholar] [CrossRef] [PubMed]
- Baum, L.; Lam, C.W.; Cheung, S.K.; Kwok, T.; Lui, V.; Tsoh, J.; Lam, L.; Leung, V.; Hui, E.; Ng, C.; et al. Six-month randomized, placebo-controlled, double-blind, pilot clinical trial of curcumin in patients with Alzheimer disease. J. Clin. Psychopharmacol. 2008, 28, 110–113. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zhai, Y.; Heng, X.; Che, F.Y.; Chen, W.; Sun, D.; Zhai, G. Oral bioavailability of curcumin: Problems and advancements. J. Drug Target 2016, 24, 694–702. [Google Scholar] [CrossRef] [PubMed]
- Wahlstrom, B.; Blennow, G. A study on the fate of curcumin in the rat. Acta Pharmacol. Toxicol. 1978, 43, 86–92. [Google Scholar] [CrossRef]
- Dulbecco, P.; Savarino, V. Therapeutic potential of curcumin in digestive diseases. World J. Gastroenterol. 2013, 19, 9256–9270. [Google Scholar] [CrossRef] [PubMed]
- Shoba, G.; Joy, D.; Joseph, T.; Majeed, M.; Rajendran, R.; Srinivas, P.S. Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers. Planta Med. 1998, 64, 353–356. [Google Scholar] [CrossRef] [PubMed]
- Moorthi, C.; Krishnan, K.; Manavalan, R.; Kathiresan, K. Preparation and characterization of curcumin-piperine dual drug loaded nanoparticles. Asian Pac. J. Trop. Biomed. 2012, 2, 841–848. [Google Scholar] [CrossRef]
- Antony, B.; Merina, B.; Iyer, V.S.; Judy, N.; Lennertz, K.; Joyal, S. A Pilot Cross-Over Study to Evaluate Human Oral Bioavailability of BCM-95CG (Biocurcumax), A Novel Bioenhanced Preparation of Curcumin. Indian J. Pharm. Sci. 2008, 70, 445–449. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Narain, U.; Mishra, R.; Misra, K. Design, development and synthesis of mixed bioconjugates of piperic acid-glycine, curcumin-glycine/alanine and curcumin-glycine-piperic acid and their antibacterial and antifungal properties. Bioorg. Med. Chem. 2005, 13, 1477–1486. [Google Scholar] [CrossRef] [PubMed]
- Comblain, F.; Sanchez, C.; Lesponne, I.; Balligand, M.; Serisier, S.; Henrotin, Y. Curcuminoids extract, hydrolyzed collagen and green tea extract synergically inhibit inflammatory and catabolic mediator’s synthesis by normal bovine and osteoarthritic human chondrocytes in monolayer. PLoS ONE 2015, 10, e0121654. [Google Scholar] [CrossRef] [PubMed]
- Maiti, K.; Mukherjee, K.; Gantait, A.; Saha, B.P.; Mukherjee, P.K. Curcumin-phospholipid complex: Preparation, therapeutic evaluation and pharmacokinetic study in rats. Int. J. Pharm. 2007, 330, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Aqil, F.; Munagala, R.; Jeyabalan, J.; Vadhanam, M.V. Bioavailability of phytochemicals and its enhancement by drug delivery systems. Cancer Lett. 2013, 334, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Haddadi, A.; Molavi, O.; Lavasanifar, A.; Lai, R.; Samuel, J. Micelles of poly(ethylene oxide)-b-poly(epsilon-caprolactone) as vehicles for the solubilization, stabilization, and controlled delivery of curcumin. J. Biomed. Mater. Res. A 2008, 86, 300–310. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Li, J.; Shi, K.; Huang, Q. Structure of modified epsilon-polylysine micelles and their application in improving cellular antioxidant activity of curcuminoids. Food Funct. 2011, 2, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Podaralla, S.; Averineni, R.; Alqahtani, M.; Perumal, O. Synthesis of novel biodegradable methoxy poly(ethylene glycol)-zein micelles for effective delivery of curcumin. Mol. Pharm. 2012, 9, 2778–2786. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Feng, R.; Sun, M.; Guo, C.; Gao, Y.; Li, L.; Zhai, G. Curcumin-loaded PLGA-PEG-PLGA triblock copolymeric micelles: Preparation, pharmacokinetics and distribution in vivo. J. Colloid Interface Sci. 2011, 354, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Kazi, K.M.; Mandal, A.S.; Biswas, N.; Guha, A.; Chatterjee, S.; Behera, M.; Kuotsu, K. Niosome: A future of targeted drug delivery systems. J. Adv. Pharm. Technol. Res. 2010, 1, 374–380. [Google Scholar] [PubMed]
- Jain, S.; Singh, P.; Mishra, V.; Vyas, S.P. Mannosylated niosomes as adjuvant-carrier system for oral genetic immunization against hepatitis B. Immunol. Lett. 2005, 101, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Yallapu, M.M.; Ebeling, M.C.; Chauhan, N.; Jaggi, M.; Chauhan, S.C. Interaction of curcumin nanoformulations with human plasma proteins and erythrocytes. Int. J. Nanomed. 2011, 6, 2779–2790. [Google Scholar]
- Kabanov, A.V.; Vinogradov, S.V. Nanogels as pharmaceutical carriers: Finite networks of infinite capabilities. Angew. Chem. Int. Ed. Engl. 2009, 48, 5418–5429. [Google Scholar] [CrossRef] [PubMed]
- Mangalathillam, S.; Rejinold, N.S.; Nair, A.; Lakshmanan, V.K.; Nair, S.V.; Jayakumar, R. Curcumin loaded chitin nanogels for skin cancer treatment via the transdermal route. Nanoscale 2012, 4, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Shen, J.; Banerjee, P.; Zhou, S. Water-dispersible multifunctional hybrid nanogels for combined curcumin and photothermal therapy. Biomaterials 2011, 32, 598–609. [Google Scholar] [CrossRef] [PubMed]
- Salomon, C.; Goycoolea, F.M.; Moerschbacher, B. Recent Trends in the Development of Chitosan-Based Drug Delivery Systems. AAPS PharmSciTech 2017, 18, 933–935. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Tang, Q.; Xu, X.; Li, N. Development and evaluation of a novel phytosome-loaded chitosan microsphere system for curcumin delivery. Int. J. Pharm. 2013, 448, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Karewicz, A.; Bielska, D.; Loboda, A.; Gzyl-Malcher, B.; Bednar, J.; Jozkowicz, A.; Dulak, J.; Nowakowska, M. Curcumin-containing liposomes stabilized by thin layers of chitosan derivatives. Colloids Surf. B Biointerfaces 2013, 109, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Omidfar, K.; Khorsand, F.; Darziani Azizi, M. New analytical applications of gold nanoparticles as label in antibody based sensors. Biosens. Bioelectron. 2013, 43, 336–347. [Google Scholar] [CrossRef] [PubMed]
- Sindhu, K.; Indra, R.; Rajaram, A.; Sreeram, K.J.; Rajaram, R. Investigations on the interaction of gold-curcumin nanoparticles with human peripheral blood lymphocytes. J. Biomed. Nanotechnol. 2011, 7, 56. [Google Scholar] [CrossRef] [PubMed]
- Sweet, M.J.; Singleton, I. Silver nanoparticles: A microbial perspective. Adv. Appl. Microbiol. 2011, 77, 115–133. [Google Scholar] [PubMed]
- Tonnesen, H.H.; Masson, M.; Loftsson, T. Studies of curcumin and curcuminoids. XXVII. Cyclodextrin complexation: Solubility, chemical and photochemical stability. Int. J. Pharm. 2002, 244, 127–135. [Google Scholar] [CrossRef]
- Torne, S.; Darandale, S.; Vavia, P.; Trotta, F.; Cavalli, R. Cyclodextrin-based nanosponges: Effective nanocarrier for tamoxifen delivery. Pharm. Dev. Technol. 2013, 18, 619–625. [Google Scholar] [CrossRef] [PubMed]
- Yadav, V.R.; Prasad, S.; Kannappan, R.; Ravindran, J.; Chaturvedi, M.M.; Vaahtera, L.; Parkkinen, J.; Aggarwal, B.B. Cyclodextrin-complexed curcumin exhibits anti-inflammatory and antiproliferative activities superior to those of curcumin through higher cellular uptake. Biochem. Pharmacol. 2010, 80, 1021–1032. [Google Scholar] [CrossRef] [PubMed]
- Longmire, M.; Choyke, P.L.; Kobayashi, H. Dendrimer-based contrast agents for molecular imaging. Curr. Top. Med. Chem. 2008, 8, 1180–1186. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, C.; Fonseca, H.B.; Jhabvala, P.; Escalon, E.A.; Melnick, S.J. Curcumin inhibits telomerase activity through human telomerase reverse transcritpase in MCF-7 breast cancer cell line. Cancer Lett. 2002, 184, 1–6. [Google Scholar] [CrossRef]
- Babaei, E.; Sadeghizadeh, M.; Hassan, Z.M.; Feizi, M.A.; Najafi, F.; Hashemi, S.M. Dendrosomal curcumin significantly suppresses cancer cell proliferation in vitro and in vivo. Int. Immunopharmacol. 2012, 12, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Ekambaram, P.; Abdul, H.S. Formulation and evaluation of solid lipid nanoparticles of ramipril. J. Young Pharm. 2011, 3, 216–220. [Google Scholar] [CrossRef] [PubMed]
- Kakkar, V.; Muppu, S.K.; Chopra, K.; Kaur, I.P. Curcumin loaded solid lipid nanoparticles: An efficient formulation approach for cerebral ischemic reperfusion injury in rats. Eur. J. Pharm. Biopharm. 2013, 85, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Gota, V.S.; Maru, G.B.; Soni, T.G.; Gandhi, T.R.; Kochar, N.; Agarwal, M.G. Safety and pharmacokinetics of a solid lipid curcumin particle formulation in osteosarcoma patients and healthy volunteers. J. Agric. Food Chem. 2010, 58, 2095–2099. [Google Scholar] [CrossRef] [PubMed]
- Dadhaniya, P.; Patel, C.; Muchhara, J.; Bhadja, N.; Mathuria, N.; Vachhani, K.; Soni, M.G. Safety assessment of a solid lipid curcumin particle preparation: Acute and subchronic toxicity studies. Food Chem. Toxicol. 2011, 49, 1834–1842. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Tu, J.; You, H.; Hu, B. Design, synthesis, and biological evaluation of novel EF24 and EF31 analogs as potential IkappaB kinase beta inhibitors for the treatment of pancreatic cancer. Drug Des. Dev. Ther. 2017, 11, 1439–1451. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Wu, X.; Xu, W.; Yang, J.; Yang, Q. Fluorescence enhancement of the silver nanoparticales—Curcumin-cetyltrimethylammonium bromide-nucleic acids system and its analytical application. J. Fluoresc. 2010, 20, 843–850. [Google Scholar] [CrossRef] [PubMed]
- Mondal, S.; Ghosh, S. Role of curcumin on the determination of the critical micellar concentration by absorbance, fluorescence and fluorescence anisotropy techniques. J. Photochem. Photobiol. B 2012, 115, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Ono, K.; Hasegawa, K.; Naiki, H.; Yamada, M. Curcumin has potent anti-amyloidogenic effects for Alzheimer’s beta-amyloid fibrils in vitro. J. Neurosci. Res. 2004, 75, 742–750. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Fiala, M.; Cashman, J.; Sayre, J.; Espinosa, A.; Mahanian, M.; Zaghi, J.; Badmaev, V.; Graves, M.C.; Bernard, G.; et al. Curcuminoids enhance amyloid-beta uptake by macrophages of Alzheimer’s disease patients. J. Alzheimer’s Dis. 2006, 10, 1–7. [Google Scholar] [CrossRef]
- Zhang, X.; Tian, Y.; Zhang, H.; Kavishwar, A.; Lynes, M.; Brownell, A.L.; Sun, H.; Tseng, Y.H.; Moore, A.; Ran, C. Curcumin analogues as selective fluorescence imaging probes for brown adipose tissue and monitoring browning. Sci. Rep. 2015, 5, 13116. [Google Scholar] [CrossRef] [PubMed]
- Jack, C.R., Jr.; Albert, M.S.; Knopman, D.S.; McKhann, G.M.; Sperling, R.A.; Carrillo, M.C.; Thies, B.; Phelps, C.H. Introduction to the recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s Dement. J. Alzheimer’s Assoc. 2011, 7, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Glenner, G.G.; Wong, C.W. Alzheimer’s disease: Initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem. Biophys. Res. Commun. 1984, 120, 885–890. [Google Scholar] [CrossRef]
- Masters, C.L.; Simms, G.; Weinman, N.A.; Multhaup, G.; McDonald, B.L.; Beyreuther, K. Amyloid plaque core protein in Alzheimer disease and Down syndrome. Proc. Natl. Acad. Sci. USA 1985, 82, 4245–4249. [Google Scholar] [CrossRef] [PubMed]
- Haass, C. Take five—BACE and the gamma-secretase quartet conduct Alzheimer’s amyloid beta-peptide generation. EMBO J. 2004, 23, 483–488. [Google Scholar] [CrossRef] [PubMed]
- Haass, C.; Selkoe, D.J. Soluble protein oligomers in neurodegeneration: Lessons from the Alzheimer’s amyloid beta-peptide. Nat. Rev. Mol. Cell Biol. 2007, 8, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Lichtenthaler, S.F.; Haass, C.; Steiner, H. Regulated intramembrane proteolysis—Lessons from amyloid precursor protein processing. J. Neurochem. 2011, 117, 779–796. [Google Scholar] [CrossRef] [PubMed]
- Kosik, K.S.; Joachim, C.L.; Selkoe, D.J. Microtubule-associated protein tau (tau) is a major antigenic component of paired helical filaments in Alzheimer disease. Proc. Natl. Acad. Sci. USA 1986, 83, 4044–4048. [Google Scholar] [CrossRef] [PubMed]
- Nukina, N.; Ihara, Y. One of the antigenic determinants of paired helical filaments is related to tau protein. J. Biochem. 1986, 99, 1541–1544. [Google Scholar] [CrossRef] [PubMed]
- Dickson, D.W. Apoptotic mechanisms in Alzheimer neurofibrillary degeneration: Cause or effect? J. Clin. Investig. 2004, 114, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Spires-Jones, T.L.; de Calignon, A.; Matsui, T.; Zehr, C.; Pitstick, R.; Wu, H.Y.; Osetek, J.D.; Jones, P.B.; Bacskai, B.J.; Feany, M.B.; et al. In vivo imaging reveals dissociation between caspase activation and acute neuronal death in tangle-bearing neurons. J. Neurosci. Off. J. Soc. Neurosci. 2008, 28, 862–867. [Google Scholar] [CrossRef] [PubMed]
- Wakasaya, Y.; Kawarabayashi, T.; Watanabe, M.; Yamamoto-Watanabe, Y.; Takamura, A.; Kurata, T.; Murakami, T.; Abe, K.; Yamada, K.; Wakabayashi, K.; et al. Factors responsible for neurofibrillary tangles and neuronal cell losses in tauopathy. J. Neurosci. Res. 2011, 89, 576–584. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Maiti, P.; Ma, Q.; Zuo, X.; Jones, M.R.; Cole, G.M.; Frautschy, S.A. Clinical development of curcumin in neurodegenerative disease. Expert Rev. Neurother. 2015, 15, 629–637. [Google Scholar] [CrossRef] [PubMed]
- Ryu, E.K.; Choe, Y.S.; Lee, K.H.; Choi, Y.; Kim, B.T. Curcumin and dehydrozingerone derivatives: Synthesis, radiolabeling, and evaluation for beta-amyloid plaque imaging. J. Med. Chem. 2006, 49, 6111–6119. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Z.; Hongmei, Z.; Lu, S.; Yu, L. Curcumin mediates presenilin-1 activity to reduce beta-amyloid production in a model of Alzheimer’s Disease. Pharmacol. Rep. 2011, 63, 1101–1108. [Google Scholar] [CrossRef]
- Mutsuga, M.; Chambers, J.K.; Uchida, K.; Tei, M.; Makibuchi, T.; Mizorogi, T.; Takashima, A.; Nakayama, H. Binding of curcumin to senile plaques and cerebral amyloid angiopathy in the aged brain of various animals and to neurofibrillary tangles in Alzheimer’s brain. J. Vet. Med. Sci. 2012, 74, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Caughey, B.; Raymond, L.D.; Raymond, G.J.; Maxson, L.; Silveira, J.; Baron, G.S. Inhibition of protease-resistant prion protein accumulation in vitro by curcumin. J. Virol. 2003, 77, 5499–5502. [Google Scholar] [CrossRef] [PubMed]
- Anand, P.; Thomas, S.G.; Kunnumakkara, A.B.; Sundaram, C.; Harikumar, K.B.; Sung, B.; Tharakan, S.T.; Misra, K.; Priyadarsini, I.K.; Rajasekharan, K.N.; et al. Biological activities of curcumin and its analogues (Congeners) made by man and Mother Nature. Biochem. Pharmacol. 2008, 76, 1590–1611. [Google Scholar] [CrossRef] [PubMed]
- Maiti, P.; Manna, J.; Veleri, S.; Frautschy, S. Molecular chaperone dysfunction in neurodegenerative diseases and effects of curcumin. Biomed. Res. Int. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Gibson, S.A.; Gao, G.D.; McDonagh, K.; Shen, S. Progress on stem cell research towards the treatment of Parkinson’s disease. Stem Cell Res. Ther. 2012, 3, 11. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos Jaques, J.A.; Ruchel, J.B.; Schlemmer, K.B.; Pimentel, V.C.; Bagatini, M.; Souza Vdo, C.; Moretto, M.B.; Morsch, V.M.; Schetinger, M.R.; Leal, D.B. Effects of curcumin on the activities of the enzymes that hydrolyse adenine nucleotides in platelets from cigarette smoke-exposed rats. Cell Biochem. Funct. 2011, 29, 630–635. [Google Scholar] [CrossRef] [PubMed]
- Monroy, A.; Lithgow, G.J.; Alavez, S. Curcumin and neurodegenerative diseases. Biofactors 2013, 39, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.L.; Fan, H.; Dang, H.Z.; Chen, X.P.; Ren, Y.; Yang, J.D.; Wang, P.W. Neuroprotective effect of curcumin to Abeta of double transgenic mice with Alzheimer’s disease. China J. Chin. Mater. Med. 2014, 39, 3846–3849. [Google Scholar]
- Wang, X.S.; Zhang, Z.R.; Zhang, M.M.; Sun, M.X.; Wang, W.W.; Xie, C.L. Neuroprotective properties of curcumin in toxin-base animal models of Parkinson’s disease: A systematic experiment literatures review. BMC Complement. Altern. Med. 2017, 17, 412. [Google Scholar] [CrossRef] [PubMed]
- Rajeswari, A.; Sabesan, M. Inhibition of monoamine oxidase-B by the polyphenolic compound, curcumin and its metabolite tetrahydrocurcumin, in a model of Parkinson’s disease induced by MPTP neurodegeneration in mice. Inflammopharmacology 2008, 16, 96–99. [Google Scholar] [CrossRef] [PubMed]
- Maiti, P.; Paladugu, L.; Dunbar, G.L. Solid lipid curcumin particles provide greater anti-amyloid, anti-inflammatory and neuroprotective effects than curcumin in the 5xFAD mouse model of Alzheimer’s disease. BMC Neurosci. 2018, 19, 7. [Google Scholar] [CrossRef] [PubMed]
- Koronyo, Y.; Biggs, D.; Barron, E.; Boyer, D.S.; Pearlman, J.A.; Au, W.J.; Kile, S.J.; Blanco, A.; Fuchs, D.T.; Ashfaq, A.; et al. Retinal amyloid pathology and proof-of-concept imaging trial in Alzheimer’s disease. JCI Insight 2017, 2. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Park, B.S.; Lee, K.G.; Choi, C.Y.; Jang, S.S.; Kim, Y.H.; Lee, S.E. Effects of naturally occurring compounds on fibril formation and oxidative stress of beta-amyloid. J. Agric. Food Chem. 2005, 53, 8537–8541. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Alloza, M.; Borrelli, L.A.; Rozkalne, A.; Hyman, B.T.; Bacskai, B.J. Curcumin labels amyloid pathology in vivo, disrupts existing plaques, and partially restores distorted neurites in an Alzheimer mouse model. J. Neurochem. 2007, 102, 1095–1104. [Google Scholar] [CrossRef] [PubMed]
- Yan, R.; Vassar, R. Targeting the beta secretase BACE1 for Alzheimer’s disease therapy. Lancet Neurol. 2014, 13, 319–329. [Google Scholar] [CrossRef]
- Narlawar, R.; Baumann, K.; Schubenel, R.; Schmidt, B. Curcumin derivatives inhibit or modulate beta-amyloid precursor protein metabolism. Neurodegener. Dis. 2007, 4, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.C.; Xu, K.; Jiang, Z.F. Curcumin-mediated neuroprotection against amyloid-beta-induced mitochondrial dysfunction involves the inhibition of GSK-3beta. J. Alzheimer’s Dis. 2012, 32, 981–996. [Google Scholar] [CrossRef] [PubMed]
- Thal, D.R. Clearance of amyloid beta-protein and its role in the spreading of Alzheimer’s disease pathology. Front. Aging Neurosci. 2015, 7, 25. [Google Scholar] [CrossRef] [PubMed]
- Frautschy, S.A.; Hu, W.; Kim, P.; Miller, S.A.; Chu, T.; Harris-White, M.E.; Cole, G.M. Phenolic anti-inflammatory antioxidant reversal of Abeta-induced cognitive deficits and neuropathology. Neurobiol. Aging 2001, 22, 993–1005. [Google Scholar] [CrossRef]
- Cole, G.M.; Morihara, T.; Lim, G.P.; Yang, F.; Begum, A.; Frautschy, S.A. NSAID and antioxidant prevention of Alzheimer’s disease: Lessons from in vitro and animal models. Ann. N. Y. Acad. Sci. 2004, 1035, 68–84. [Google Scholar] [CrossRef] [PubMed]
- Mondragon-Rodriguez, S.; Perry, G.; Zhu, X.; Moreira, P.I.; Acevedo-Aquino, M.C.; Williams, S. Phosphorylation of tau protein as the link between oxidative stress, mitochondrial dysfunction, and connectivity failure: Implications for Alzheimer’s disease. Oxid. Med. Cell. Longev. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Stoothoff, W.H.; Johnson, G.V. Tau phosphorylation: Physiological and pathological consequences. Biochim. Biophys. Acta 2005, 1739, 280–297. [Google Scholar] [CrossRef] [PubMed]
- Rane, J.S.; Bhaumik, P.; Panda, D. Curcumin Inhibits Tau Aggregation and Disintegrates Preformed Tau Filaments in vitro. J. Alzheimer’s Dis. 2017, 60, 999–1014. [Google Scholar] [CrossRef] [PubMed]
- Gella, A.; Durany, N. Oxidative stress in Alzheimer disease. Cell Adhes. Migr. 2009, 3, 88–93. [Google Scholar] [CrossRef]
- Venigalla, M.; Gyengesi, E.; Munch, G. Curcumin and Apigenin—Novel and promising therapeutics against chronic neuroinflammation in Alzheimer’s disease. Neural Regen. Res. 2015, 10, 1181–1185. [Google Scholar] [PubMed]
- Liu, Z.J.; Li, Z.H.; Liu, L.; Tang, W.X.; Wang, Y.; Dong, M.R.; Xiao, C. Curcumin Attenuates Beta-Amyloid-Induced Neuroinflammation via Activation of Peroxisome Proliferator-Activated Receptor-Gamma Function in a Rat Model of Alzheimer’s Disease. Front. Pharmacol. 2016, 7, 261. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Tian, Y.; Zhang, C.; Tian, X.; Ross, A.W.; Moir, R.D.; Sun, H.; Tanzi, R.E.; Moore, A.; Ran, C. Near-infrared fluorescence molecular imaging of amyloid beta species and monitoring therapy in animal models of Alzheimer’s disease. Proc. Natl. Acad. Sci. USA 2015, 112, 9734–9739. [Google Scholar] [CrossRef] [PubMed]
- Ran, C.; Xu, X.; Raymond, S.B.; Ferrara, B.J.; Neal, K.; Bacskai, B.J.; Medarova, Z.; Moore, A. Design, synthesis, and testing of difluoroboron-derivatized curcumins as near-infrared probes for in vivo detection of amyloid-beta deposits. J. Am. Chem. Soc. 2009, 131, 15257–15261. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Tian, Y.; Yuan, P.; Li, Y.; Yaseen, M.A.; Grutzendler, J.; Moore, A.; Ran, C. A bifunctional curcumin analogue for two-photon imaging and inhibiting crosslinking of amyloid beta in Alzheimer’s disease. Chem. Commun. 2014, 50, 11550–11553. [Google Scholar] [CrossRef] [PubMed]
- Alexander, G.E. Biology of Parkinson’s disease: Pathogenesis and pathophysiology of a multisystem neurodegenerative disorder. Dialogues Clin. Neurosci. 2004, 6, 259–280. [Google Scholar] [PubMed]
- Dauer, W.; Przedborski, S. Parkinson’s disease: Mechanisms and models. Neuron 2003, 39, 889–909. [Google Scholar] [CrossRef]
- Perez, C.A.; Tong, Y.; Guo, M. Iron Chelators as Potential Therapeutic Agents for Parkinson’s Disease. Curr. Bioact. Compd. 2008, 4, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.F.; Zhang, Y.J.; Zhou, H.Y.; Wang, H.M.; Tian, L.P.; Liu, J.; Ding, J.Q.; Chen, S. Curcumin ameliorates the neurodegenerative pathology in A53T alpha-synuclein cell model of Parkinson’s disease through the downregulation of mTOR/p70S6K signaling and the recovery of macroautophagy. J. Neuroimmune Pharmacol. 2013, 8, 356–369. [Google Scholar] [CrossRef] [PubMed]
- Khatri, D.K.; Juvekar, A.R. Kinetics of Inhibition of Monoamine Oxidase Using Curcumin and Ellagic Acid. Pharmacogn. Mag. 2016, 12, S116–S120. [Google Scholar] [PubMed]
- Nam, S.M.; Choi, J.H.; Yoo, D.Y.; Kim, W.; Jung, H.Y.; Kim, J.W.; Yoo, M.; Lee, S.; Kim, C.J.; Yoon, Y.S.; et al. Effects of curcumin (Curcuma longa) on learning and spatial memory as well as cell proliferation and neuroblast differentiation in adult and aged mice by upregulating brain-derived neurotrophic factor and CREB signaling. J. Med. Food 2014, 17, 641–649. [Google Scholar] [CrossRef] [PubMed]
- Jayaraj, R.L.; Elangovan, N.; Manigandan, K.; Singh, S.; Shukla, S. CNB-001 a novel curcumin derivative, guards dopamine neurons in MPTP model of Parkinson’s disease. Biomed. Res. Int. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Landles, C.; Bates, G.P. Huntingtin and the molecular pathogenesis of Huntington’s disease. Fourth in molecular medicine review series. EMBO Rep. 2004, 5, 958–963. [Google Scholar] [CrossRef] [PubMed]
- Walker, F.O. Huntington’s Disease. Semin. Neurol. 2007, 27, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Han, I.; You, Y.; Kordower, J.H.; Brady, S.T.; Morfini, G.A. Differential vulnerability of neurons in Huntington’s disease: The role of cell type-specific features. J. Neurochem. 2010, 113, 1073–1091. [Google Scholar] [CrossRef] [PubMed]
- Metzler, M.; Chen, N.; Helgason, C.D.; Graham, R.K.; Nichol, K.; McCutcheon, K.; Nasir, J.; Humphries, R.K.; Raymond, L.A.; Hayden, M.R. Life without huntingtin: Normal differentiation into functional neurons. J. Neurochem. 1999, 72, 1009–1018. [Google Scholar] [CrossRef] [PubMed]
- Schulte, J.; Littleton, J.T. The biological function of the Huntingtin protein and its relevance to Huntington’s Disease pathology. Curr. Trends Neurol. 2011, 5, 65–78. [Google Scholar] [PubMed]
- Zuccato, C.; Marullo, M.; Vitali, B.; Tarditi, A.; Mariotti, C.; Valenza, M.; Lahiri, N.; Wild, E.J.; Sassone, J.; Ciammola, A.; et al. Brain-derived neurotrophic factor in patients with Huntington’s disease. PLoS ONE 2011, 6, e22966. [Google Scholar] [CrossRef] [PubMed]
- Aronin, N.; Chase, K.; Young, C.; Sapp, E.; Schwarz, C.; Matta, N.; Kornreich, R.; Landwehrmeyer, B.; Bird, E.; Beal, M.F.; et al. CAG expansion affects the xpression of mutant Huntingtin in the Huntington’s disease brain. Neuron 1995, 15, 1193–1201. [Google Scholar] [CrossRef]
- Miller, B.R.; Bezprozvanny, I. Corticostriatal circuit dysfunction in Huntington’s disease: Intersection of glutamate, dopamine and calcium. Future Neurol. 2010, 5, 735–756. [Google Scholar] [CrossRef] [PubMed]
- Mangiarini, L.; Sathasivam, K.; Seller, M.; Cozens, B.; Harper, A.; Hetherington, C.; Lawton, M.; Trottier, Y.; Lehrach, H.; Davies, S.W.; et al. Exon 1 of the HD gene with an expanded CAG repeat is sufficient to cause a progressive neurological phenotype in transgenic mice. Cell 1996, 87, 493–506. [Google Scholar] [CrossRef]
- Spires, T.L.; Grote, H.E.; Garry, S.; Cordery, P.M.; Van Dellen, A.; Blakemore, C.; Hannan, A.J. Dendritic spine pathology and deficits in experience-dependent dendritic plasticity in R6/1 Huntington’s disease transgenic mice. Eur. J. Neurosci. 2004, 19, 2799–2807. [Google Scholar] [CrossRef] [PubMed]
- Nithianantharajah, J.; Barkus, C.; Murphy, M.; Hannan, A.J. Gene-environment interactions modulating cognitive function and molecular correlates of synaptic plasticity in Huntington’s disease transgenic mice. Neurobiol. Dis. 2008, 29, 490–504. [Google Scholar] [CrossRef] [PubMed]
- Taylor-Robinson, S.D.; Weeks, R.A.; Sargentoni, J.; Marcus, C.D.; Bryant, D.J.; Harding, A.E.; Brooks, D.J. Evidence for glutamate excitotoxicity in Huntington’s disease with proton magnetic resonance spectroscopy. Lancet 1994, 343, 1170. [Google Scholar] [CrossRef]
- Quintanilla, R.A.; Johnson, G.V. Role of mitochondrial dysfunction in the pathogenesis of Huntington’s disease. Brain Res. Bull. 2009, 80, 242–247. [Google Scholar] [CrossRef] [PubMed]
- Rubinsztein, D.C.; Carmichael, J. Huntington’s disease: Molecular basis of neurodegeneration. Expert Rev. Mol. Med. 2003, 5, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Sandhir, R.; Yadav, A.; Mehrotra, A.; Sunkaria, A.; Singh, A.; Sharma, S. Curcumin nanoparticles attenuate neurochemical and neurobehavioral deficits in experimental model of Huntington’s disease. Neuromol. Med. 2014, 16, 106–118. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Dou, W.; Zheng, Y.; Wen, Q.; Qin, M.; Wang, X.; Tang, H.; Zhang, R.; Lv, D.; Wang, J.; et al. Curcumin upregulates Nrf2 nuclear translocation and protects rat hepatic stellate cells against oxidative stress. Mol. Med. Rep. 2016, 13, 1717–1724. [Google Scholar] [CrossRef] [PubMed]
- Bekinschtein, P.; Cammarota, M.; Katche, C.; Slipczuk, L.; Rossato, J.I.; Goldin, A.; Izquierdo, I.; Medina, J.H. BDNF is essential to promote persistence of long-term memory storage. Proc. Natl. Acad. Sci. USA 2008, 105, 2711–2716. [Google Scholar] [CrossRef] [PubMed]
- Dikshit, P.; Goswami, A.; Mishra, A.; Nukina, N.; Jana, N.R. Curcumin enhances the polyglutamine-expanded truncated N-terminal huntingtin-induced cell death by promoting proteasomal malfunction. Biochem. Biophys. Res. Commun. 2006, 342, 1323–1328. [Google Scholar] [CrossRef] [PubMed]
- Dikshit, P.; Goswami, A.; Mishra, A.; Chatterjee, M.; Jana, N.R. Curcumin induces stress response, neurite outgrowth and prevent NF-kappaB activation by inhibiting the proteasome function. Neurotox. Res. 2006, 9, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Jana, N.R.; Dikshit, P.; Goswami, A.; Nukina, N. Inhibition of proteasomal function by curcumin induces apoptosis through mitochondrial pathway. J. Biol. Chem. 2004, 279, 11680–11685. [Google Scholar] [CrossRef] [PubMed]
- Prusiner, S.B. The prion diseases. Brain Pathol. 1998, 8, 499–513. [Google Scholar] [CrossRef] [PubMed]
- Brundin, P.; Melki, R.; Kopito, R. Prion-like transmission of protein aggregates in neurodegenerative diseases. Nat. Rev. Mol. Cell Biol. 2010, 11, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Gambetti, P.; Kong, Q.; Zou, W.; Parchi, P.; Chen, S.G. Sporadic and familial CJD: Classification and characterisation. Br. Med. Bull. 2003, 66, 213–239. [Google Scholar] [CrossRef] [PubMed]
- Shang, H.S.; Chang, C.H.; Chou, Y.R.; Yeh, M.Y.; Au, M.K.; Lu, H.F.; Chu, Y.L.; Chou, H.M.; Chou, H.C.; Shih, Y.L.; et al. Curcumin causes DNA damage and affects associated protein expression in HeLa human cervical cancer cells. Oncol. Rep. 2016, 36, 2207–2215. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Jia, L.; Zhou, H.M.; Liu, Y.; Zhong, L.F. Mitochondrial and nuclear DNA damage induced by curcumin in human hepatoma G2 cells. Toxicol. Sci. 2006, 91, 476–483. [Google Scholar] [CrossRef] [PubMed]
- Dance-Barnes, S.T.; Kock, N.D.; Moore, J.E.; Lin, E.Y.; Mosley, L.J.; D’Agostino, R.B., Jr.; McCoy, T.P.; Townsend, A.J.; Miller, M.S. Lung tumor promotion by curcumin. Carcinogenesis 2009, 30, 1016–1023. [Google Scholar] [CrossRef] [PubMed]
- Ravindran, J.; Prasad, S.; Aggarwal, B.B. Curcumin and cancer cells: How many ways can curry kill tumor cells selectively? AAPS J. 2009, 11, 495–510. [Google Scholar] [CrossRef] [PubMed]
- Liddle, M.; Hull, C.; Liu, C.; Powell, D. Contact urticaria from curcumin. Dermatitis 2006, 17, 196–197. [Google Scholar] [CrossRef] [PubMed]
- Burgos-Moron, E.; Calderon-Montano, J.M.; Salvador, J.; Robles, A.; Lopez-Lazaro, M. The dark side of curcumin. Int. J. Cancer 2010, 126, 1771–1775. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.C.; Ku, S.K.; Bae, J.S. Anticoagulant activities of curcumin and its derivative. BMB Rep. 2012, 45, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.A.; Euden, S.A.; Platton, S.L.; Cooke, D.N.; Shafayat, A.; Hewitt, H.R.; Marczylo, T.H.; Morgan, B.; Hemingway, D.; Plummer, S.M.; et al. Phase I clinical trial of oral curcumin: Biomarkers of systemic activity and compliance. Clin. Cancer Res. 2004, 10, 6847–6854. [Google Scholar] [CrossRef] [PubMed]
- Rasyid, A.; Lelo, A. The effect of curcumin and placebo on human gall-bladder function: An ultrasound study. Aliment. Pharmacol. Ther. 1999, 13, 245–249. [Google Scholar] [CrossRef] [PubMed]
- Rasyid, A.; Rahman, A.R.; Jaalam, K.; Lelo, A. Effect of different curcumin dosages on human gall bladder. Asia Pac. J. Clin. Nutr. 2002, 11, 314–318. [Google Scholar] [CrossRef] [PubMed]
- Ringman, J.M.; Frautschy, S.A.; Teng, E.; Begum, A.N.; Bardens, J.; Beigi, M.; Gylys, K.H.; Badmaev, V.; Heath, D.D.; Apostolova, L.G.; et al. Oral curcumin for Alzheimer’s disease: Tolerability and efficacy in a 24-week randomized, double blind, placebo-controlled study. Alzheimer’s Res. Ther. 2012, 4, 43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hishikawa, N.; Takahashi, Y.; Amakusa, Y.; Tanno, Y.; Tuji, Y.; Niwa, H.; Murakami, N.; Krishna, U.K. Effects of turmeric on Alzheimer’s disease with behavioral and psychological symptoms of dementia. Ayu 2012, 33, 499–504. [Google Scholar] [CrossRef] [PubMed]
- Goozee, K.G.; Shah, T.M.; Sohrabi, H.R.; Rainey-Smith, S.R.; Brown, B.; Verdile, G.; Martins, R.N. Examining the potential clinical value of curcumin in the prevention and diagnosis of Alzheimer’s disease. Br. J. Nutr. 2016, 115, 449–465. [Google Scholar] [CrossRef] [PubMed]
- Small, G.W.; Siddarth, P.; Li, Z.; Miller, K.J.; Ercoli, L.; Emerson, N.D.; Martinez, J.; Wong, K.P.; Liu, J.; Merrill, D.A.; et al. Memory and Brain Amyloid and Tau Effects of a Bioavailable Form of Curcumin in Non-Demented Adults: A Double-Blind, Placebo-Controlled 18-Month Trial. Am. J. Geriatr. Psychiatry 2018, 26, 266–277. [Google Scholar] [CrossRef] [PubMed]
- Cox, K.H.; Pipingas, A.; Scholey, A.B. Investigation of the effects of solid lipid curcumin on cognition and mood in a healthy older population. J. Psychopharmacol. 2015, 29, 642–651. [Google Scholar] [CrossRef] [PubMed]






















| Characteristics | Cur-I | Cur-II | Cur-III |
|---|---|---|---|
| Common Name | Cur | DemethoxyCur | BisdemethoxyCur |
| Chemical Name | Dicinnamoyl methane | 4-OH cinnamoyl methane | Bis-4-OH cinnamoyl methane |
| Color | Bright orange-yellow | Bright orange-yellow | Bright orange-yellow |
| Amount Present (%) | 77 | 17 | 3 |
| Molecular Mass (g/mol) | 368.4 | 338.0 | 308.1 |
| Melting Point (°C) | 183.0–186.0 | 172.5–174.5 | 224.0 |
| Neutral Solvent (water) | Poorly soluble | Poorly soluble | Poorly soluble |
| Solubility in Organic Solvents | Soluble | Soluble | Soluble |
| Solubility in Hexane or Ether | Insoluble | Insoluble | Insoluble |
| Excitation/Emission in | 420/530 nm | 420/530 nm | 420/530 nm |
| Excitation/Emission in Alcohol | 536–560 nm | Unknown | Unknown |
| Proteins | Diseases | Nature of Binding of Cur | Outcomes | Ref. |
|---|---|---|---|---|
| Aβ | AD | With amino acid 16–21 of Aβ | Inhibits oligomer and fibril formation, thus decrease Aβ induced neurotoxicity | [9,13,34] |
| Tau | Tauopathies, AD | In the microtubule-binding region of tau | Inhibits phosphorylated tau, thus decrease neurofibrillary tangle | [12,37] |
| α-Syn | PD | In the hydrophobic no Aβ component region | Inhibits α-syn oligomers and fibril formation, thus decrease α-Syn induced oxidative damage | [35,39] |
| HTT | HD | Unknown | Lower doses (nM) decrease HTT aggregates | [36,40] |
| Prion | Prion | α-Helical intermediate and to the amyloid form of prion protein | Inhibits PrPsc accumulation | [38,41] |
| Materials | Compounds Used with Cur |
|---|---|
| Adjuvant | Piperine |
| Bio-conjugates | Turmeric oil, glycine, alanine, EGCG |
| Lipids | Phospholipid, liposome, oil body emulsion |
| Nanoparticles | GMO, Chitosan, cyclodextrin, PLGA, silica, PHEMA, gold, silver, casein, orange gel-based nano emulsion, dendrimer, solid lipid particles |
| Protein | BSA, soy protein isolated |
| Others | Hyaluronic acid, hydrogel, polymer, PEG-PEI emulsion, polymer encapsulated, beta-lactoglobulin |
| Parameters | Tmax | Cmax | Ke | t1/2 | AUC (o.inf) | Cl (Observed)/F | Vz (observed)/F |
|---|---|---|---|---|---|---|---|
| Cur | 2 | 149.8 | 0.296 | 2.63 | 461.86 | 0.006735 | 0.026362 |
| BCM—95 RCG | 3.44 | 456.88 | 0.26 | 4.96 | 3201.28 | 0.001682 | 0.006784 |
| Nanoparticles | Schematic Diagram | Shape | Size (nm) | Methods | Outcome |
|---|---|---|---|---|---|
| Liposome |  | Globular | 25–205 | In vitro, in vivo (dogs & mice) | Increased solubility, tissue distribution, and stability |
| Micelles |  | Spherical | 10–100 | In vitro, In vivo (mice) | Increased solubility and bioavailability; Improved anti-oxidative properties |
| Noisome |  | Lamellar | 190–1140 | In vitro, In vivo (snake and mice) | Increased skin penetration; Prolonged delivery system |
| Nanogel network |  | Cross-linked polymer | 10–200 | In vitro | Increased stability, fluorescence effects, developed bioavailability, get better control release; Prolonged half-life |
| Chitosan |  | Linear polysaccharide composed | 100–250 | In vitro, In vivo (rats and mice) | Improved chemical stability, improved antioxidant effects; Prolonged blood circulation |
| Gold |  | Globular | 200–250 | In vitro | Improved solubility; Enhanced antioxidant |
| Silver |  | Film layer | ~15 | In vitro | Improved wound healing; Increased antiviral and anticancer effects |
| Cyclodextrin |  | cyclic oligomers of glucose oligosaccharide | In vitro | Improve stability and bioavailability of Cur | |
| Dendrimer |  | Globular polymer | 15–150 | In vitro, In vivo (mice) | Improved stability; Increased antitumor and anti-proliferative effects |
| Solid lipid |  | Spherical | 50–1000 | In vitro, In vivo (rat and mice) | Prolonged circulation of blood; Increased anti-inflammatory effects; Improved brain delivery |
| Actions | Mechanisms | References |
|---|---|---|
| Anti-amyloid properties | Binds with Aβ and prevent its oligomerization and fibril formation | [9,112,127] |
| Inhibition of Aβ production | Inhibits activities of β-secretase (BACE), inhibiting amyloid precursor protein (APP) processing pathway | [33,128] |
| Aβ clearance | Stimulates phagocytosis, thus decrease Aβ-plaques | [9,10,51] |
| Inhibition of NFTs | Binds with NFTs and inhibits tau phosphorylation (pTau) | [129] |
| Inhibition of other amyloids | Binds with α-synuclein in PD, huntingtin in HD, and prion aggregates in prion diseases | [35,130] |
| Potent antioxidant | Scavenges ROS/RONS, increase antioxidant levels, decreases lipid peroxidation, chelates toxic metals | [10,51,131] |
| Anti-inflammatory activities | Downregulates NF-κB, COX-2, 5-LOX, TNFα, IL-1, IL-6. | [10,51] |
| Regulates activity of molecular chaperones | Restores levels of heat shock proteins (HSP90, 70, 60, 40, HSC70), proteasome system | [132] |
| Enhance NGF, BDNF, GDNF, neurogenesis and synaptogenesis | Increase expression of BDNF, NGF, GDNF and can promote neurogenesis, and synaptogenesis | [10,133] |
| Improving cerebral circulation | Inhibits inflammation of brain vasculature leading to improvement of overall blood supply, reduces platelet adhesion in the brain microvascular endothelial cells | [69,134] |
| Animal Models | Dose and Duration of Treatment | Disease | Outcomes | Ref. |
|---|---|---|---|---|
| Sprague-Dawley rat | Diet, 500 and 2000 ppm, 2 months | AD (Aβ ICV infusion) | Decrease spatial memory deficit, oxidative damage, microgliosis | [135] |
| 3XTg-AD mice | Diet, 555 ppm, 2 months | AD (Aβ overexpression) | Decreased Aβ plaque deposition | [12] |
| APPswe/PS1dE9 mice | Diet, 160 and 5000 ppm, 6 months | AD (Aβ overexpression) | Reduced hippocampal Aβ40/Aβ42 levels | [136] |
| APPswe/PS1dE9 mice | AD (Aβ overexpression) | Improved spatial memory and decreased Aβ40/Aβ42 levels | [114] | |
| Tg2576 mice | Diet, 500 ppm, 4 m | AD (Aβ overexpression) | Decrease cell death, Aβ-plaques, prevent fibril formation | [9] |
| PS-1dE9 mice | IV, 7.5 mg/kg/day, 7 days | AD (Aβ overexpression) | Increased restoration of distorted neuritis, plaque disruption | [135] |
| Kunming mice | PO, 200 mg/kg, 45 days | AD (AlCl3, d-galactose) | Decrease spatial memory deficit | [135] |
| Sprague-Dawley rat | PO, 50 mg/kg, 4 days | PD (6-OHDA) | Improve TH+ cell numbers | [135] |
| ICR mice | IP, 50 mg/kg, 3 times | PD (MPTP) | Decreased oxidative damage, increase dopaminergic neurons | [137] |
| Swiss albino mice | IP, 80 mg/kg, 7 days | PD (MPTP) | Decreased MAO-B | [135,138] |
| CAG140 mice | Diet, 555 ppm, 2m | HD (knock in) | Decreased huntingtin aggregation, increase rearing, decrease climbing | [40] |
| 5XFAD | IP, 100 mg/kg, 2–5 days | AD (transgenic) | Decreased Aβ plaque, prevent cell death | [108] |
| Parameter | Side Effects | Ref. |
|---|---|---|
| General effects | Gastrointestinal discomfort, chest tightness, skin rashes, and swollen skin, allergic reactions or dermatitis, nausea, and diarrhea | [192] |
| Blood clotting | Slow down blood clotting process | [193] |
| Gall bladder | Increase gallstones contraction and increase bile duct obstruction | [192] |
| Pregnancy and postnatal complications | Stimulate the uterus or promote a menstrual period. Breast feeding women not recommended | [8] |
| Stomach problems | Increased stomach acid secretion if taken with antacid drugs | [8] |
| Study ID | Curcumin Molecule | Cohort | Dose | Duration | Outcomes | Ref. |
|---|---|---|---|---|---|---|
| Baum et al. NCT00164749 | Cur + gingko | AD:50+ year, n= 30 | 1, 4 g/day | 6 months | No differences in Aβ levels between treatments or MMSE scores | [71] |
| Ringman et al. (ACT00099710) | Cur C3 complex | Mid/Moderate AD, 49 y+, n = 30 | 2, 4 g/day | 24 weeks | No differences detected between treatment groups in biomarkers measured, low bioavailability | [197] |
| Hishikawa et al. | Tumeric capsule | Severe AD, n = 3 | 100 mg/day | 12 months tested after 12 weeks | MMSE and NPIQ; score on NPIQ decreased significantly, MMSE increased in 1/3 | [198] |
| Poncha (NCT01001637) | Longvida | Moderate-severe AD, 50–80 y, n = 160 | 2, 3 g/twice daily | 2 months | Efficacy and safety: blood and cognition | [199] |
| Martin and Goozee (ACTRN12613000681752) | Biocurucmax (BCM-95) | Retirement, healthy, 65–95, n = 100 | 500 mg/thrice/day | 12 months | Cognition, blood biomarkers, brain imaging, retinal imaging | [199] |
| Martin (ACTRN12611000437965) | Biocurucmax (BCM-95) | Community living, healthy, 55–75, n = 100 | 500 mg/thrice/day | 12 months | Cognition, blood biomarkers, life style, brain imaging | [199] |
| Small et al. (NCT01383161) | Tetracurcumin CR-031PTM | MCI, normal aging, n = 132 | 90 mg/twice/day | 18 months | Cognition, blood genetic profile | [200] |
| Frautschy (NCT018811381) | Longvida and Yoga | Subjective cognitive complainers, 55–90, n = 80 | 400 mg/twice/daily | 6 months | Biochemistry, cognition, brain imaging | [199] |
| Cox et al. (ACTRN12612001027808) | LongvidaTM | Healthy and cognitive decline, 65–80, n= 60 | 400, 800 mg/daily | 4weeks 8 weeks | Cognition, mood and anxiety, blood biomarkers, MRI | [201] |
| NCT00595582 | Curcumin bioperine | MCI, 55–85, n = 10 | 900 mg/twice/daily | 24 months | Cognition and size of metabolic lesion by PET | [199] |
| ACTRN12614001024639 | BCM-95 | Healthy and MCI, 65–90 years, n = 48 | 500 mg/twice/daily | 3 months | Gene regulation and expression, and cognition | [199] |
| ACTRN12613000367741 | LongvidaTM | Healthy, MCI, mild/moderate AD, 50 years, n = 200 | 20 g/daily | 7 days | Diagnostics, Curcumin fluorescent retinal imaging of Aβ plaques | [199] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maiti, P.; Dunbar, G.L. Use of Curcumin, a Natural Polyphenol for Targeting Molecular Pathways in Treating Age-Related Neurodegenerative Diseases. Int. J. Mol. Sci. 2018, 19, 1637. https://doi.org/10.3390/ijms19061637
Maiti P, Dunbar GL. Use of Curcumin, a Natural Polyphenol for Targeting Molecular Pathways in Treating Age-Related Neurodegenerative Diseases. International Journal of Molecular Sciences. 2018; 19(6):1637. https://doi.org/10.3390/ijms19061637
Chicago/Turabian StyleMaiti, Panchanan, and Gary L. Dunbar. 2018. "Use of Curcumin, a Natural Polyphenol for Targeting Molecular Pathways in Treating Age-Related Neurodegenerative Diseases" International Journal of Molecular Sciences 19, no. 6: 1637. https://doi.org/10.3390/ijms19061637