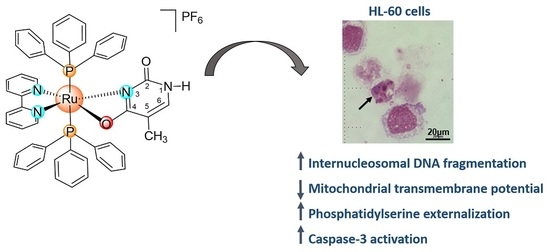
Ru(II)-Thymine Complex Causes Cell Growth Inhibition and Induction of Caspase-Mediated Apoptosis in Human Promyelocytic Leukemia HL-60 Cells
Abstract
:1. Introduction
2. Results and Discussion
2.1. The [Ru(PPh3)2(Thy)(bipy)]PF6 Complex Exhibits Potent Cytotoxicity in Different Cancer Cells
2.2. The [Ru(PPh3)2(Thy)(bipy)]PF6 Complex Triggers Caspase-Mediated Apoptosis in HL-60 Cells
3. Materials and Methods
3.1. Synthesis of the [Ru(PPh3)2(Thy)(bipy)]PF6 Complex
3.2. Cells and Cytotoxicity Assay
3.3. Flow Cytometric Assays
3.4. Statistical Analysis
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Torre, L.A.; Bray, F.; Siegel, R.L.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. Global cancer statistics, 2012. Cancer J. Clin. 2015, 65, 87–108. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Shen, T.; Yang, H.; Gu, W. Ruthenium complexes induce HepG2 human hepatocellular carcinoma cell apoptosis and inhibit cell migration and invasion through regulation of the Nrf2 pathway. Int. J. Mol. Sci. 2016, 17, 775. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Chen, Y.; Liu, J.; Huang, H.; Guan, R.; Ji, L.; Chao, H. Ruthenium(II) complexes with 2-phenylimidazo[4,5-f][1,10]phenanthroline derivatives that strongly combat cisplatin-resistant tumor cells. Sci. Rep. 2016, 6, 19449. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Liu, Y.; Liu, Y.; Qin, X.; Zhou, Y.; Liu, J. Dinuclear ruthenium complexes display loop isomer selectivity to c-MYC DNA G-quadriplex and exhibit anti-tumour activity. J. Inorg. Biochem. 2016, 156, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Costa, C.O.D.; Araujo-Neto, J.H.; Baliza, I.R.S.; Dias, R.B.; Valverde, L.F.; Vidal, M.T.A.; Sales, C.B.; Rocha, C.A.G.; Moreira, D.R.M.; Soares, M.B.P.; et al. Novel piplartine-containing ruthenium complexes: Synthesis, cell growth inhibition, apoptosis induction and ROS production on HCT116 cells. Oncotarget 2017, 8, 104367–104392. [Google Scholar]
- Siewert, B.; Langerman, M.; Hontani, Y.; Kennis, J.T.M.; van Rixel, V.H.S.; Limburg, B.; Siegler, M.A.; Talens Saez, V.; Kieltyka, R.E.; Bonnet, S. Turning on the red phosphorescence of a [Ru(tpy)(bpy)(Cl)]Cl complex by amide substitution: Self-aggregation, toxicity, and cellular localization of an emissive ruthenium-based amphiphile. Chem. Commun. 2017, 53, 11126–11129. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, K.M.; Liany, L.D.; Corrêa, R.S.; Deflon, V.M.; Cominetti, M.R.; Batista, A.A. Selective Ru(II)/lawsone complexes inhibiting tumor cell growth by apoptosis. J. Inorg. Biochem. 2017, 176, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Peng, F.; Zhang, Y.; Li, B.; She, J.; Jie, X.; Zou, Z.; Chen, M.; Chen, L. Synthesis, characterization, cellular uptake and apoptosis-inducing properties of two highly cytotoxic cyclometalated ruthenium(II) β-carboline complexes. Eur. J. Med. Chem. 2017, 140, 104–117. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, N.C.; Neves, S.P.; Dias, R.B.; Valverde, L.F.; Sales, C.B.; Rocha, C.A.G.; Soares, M.B.P.; Santos, E.R.; Oliveira, R.M.M.; Carlos, R.M.; et al. A novel ruthenium complex with xanthoxylin induces S-phase arrest and causes ERK1/2-mediated apoptosis in HepG2 cells through a p53-independent pathway. Cell Death Dis. 2018, 9, 79. [Google Scholar] [CrossRef] [PubMed]
- Silva, V.R.; Corrêa, R.S.; Santos, L.S.; Soares, M.B.P.; Batista, A.A.; Bezerra, D.P. A ruthenium-based 5-fluorouracil complex with enhanced cytotoxicity and apoptosis induction action in HCT116 cells. Sci. Rep. 2018, 8, 288. [Google Scholar] [CrossRef] [PubMed]
- Lentz, F.; Drescher, A.; Lindauer, A.; Henke, M.; Hilger, R.A.; Hartinger, C.G.; Scheulen, M.E.; Dittrich, C.; Keppler, B.K.; Jaehde, U.; et al. Pharmacokinetics of a novel anticancer ruthenium complex (KP1019, FFC14A) in a phase I dose-escalation study. Anticancer Drugs 2009, 20, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Leijen, S.; Burgers, S.A.; Baas, P.; Pluim, D.; Tibben, M.; van Werkhoven, E.; Alessio, E.; Sava, G.; Beijnen, J.H.; Schellens, J.H. Phase I/II study with ruthenium compound NAMI-A and gemcitabine in patients with non-small cell lung cancer after first line therapy. Investig. New Drugs 2015, 33, 201–214. [Google Scholar] [CrossRef] [PubMed]
- Correa, R.S.; Freire, V.; Barbosa, M.I.F.; Bezerra, D.P.; Bomfim, L.M.; Moreira, D.R.M.; Soares, M.B.P.; Ellena, J.; Batista, A.A. Ru(II)-thyminate complexes: New metallodrug candidates against tumor cells. New J. Chem. 2018, 42, 6794–6802. [Google Scholar] [CrossRef]
- Yun, S.H.; Sim, E.H.; Han, S.H.; Kim, T.R.; Ju, M.H.; Han, J.Y.; Jeong, J.S.; Kim, S.H.; Silchenko, A.S.; Stonik, V.A.; et al. In vitro and in vivo anti-leukemic effects of cladoloside C2 are mediated by activation of Fas/ceramide synthase 6/p38 kinase/c-Jun NH2-terminal kinase/caspase-8. Oncotarget 2017, 9, 495–511. [Google Scholar] [PubMed]
- Abu, E.l.; Maaty, M.A.; Almouhanna, F.; Wölfl, S. Expression of TXNIP in cancer cells and regulation by 1,25(OH)2D3: Is it really the vitamin D3 upregulated protein? Int. J. Mol. Sci. 2018, 19, 796. [Google Scholar]
- Calgarotto, A.K.; Maso, V.; Junior, G.C.F.; Nowill, A.E.; Filho, P.L.; Vassallo, J.; Saad, S.T.O. Antitumor activities of quercetin and green tea in xenografts of human leukemia HL60 cells. Sci. Rep. 2018, 8, 3459. [Google Scholar] [CrossRef] [PubMed]
- Bezerra, D.P.; Militão, G.C.; Castro, F.O.; Pessoa, C.; Moraes, M.O.; Silveira, E.R.; Lima, M.A.; Elmiro, F.J.; Costa-Lotufo, L.V. Piplartine induces inhibition of leukemia cell proliferation triggering both apoptosis and necrosis pathways. Toxicol. In Vitro 2007, 21, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Magalhães, H.I.; Wilke, D.V.; Bezerra, D.P.; Cavalcanti, B.C.; Rotta, R.; de Lima, D.P.; Beatriz, A.; Moraes, M.O.; Diniz-Filho, J.; Pessoa, C. (4-Methoxyphenyl)(3,4,5-trimethoxyphenyl)methanone inhibits tubulin polymerization, induces G2/M arrest, and triggers apoptosis in human leukemia HL-60 cells. Toxicol. Appl. Pharmacol. 2013, 272, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Li, G.; Peng, F.; Jie, X.; Dongye, G.; Cai, K.; Feng, R.; Li, B.; Zeng, Q.; Lun, K.; et al. The induction of autophagy against mitochondria-mediated apoptosis in lung cancer cells by a ruthenium (II) imidazole complex. Oncotarget 2016, 7, 80716–80734. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, L.; Shi, Z.; Zhao, S.; Wang, F.T.; Zhou, T.T.; Liu, B.; Bao, J.K. Programmed cell death pathways in cancer: A review of apoptosis, autophagy and programmed necrosis. Cell Prolif. 2012, 45, 487–498. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, L.; Vitale, I.; Aaronson, S.A.; Abrams, J.M.; Adam, D.; Agostinis, P.; Alnemri, E.S.; Altucci, L.; Amelio, I.; Andrews, D.W.; et al. Molecular mechanisms of cell death: Recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018, 25, 486–541. [Google Scholar] [CrossRef] [PubMed]
- Van Engeland, M.; Nieland, L.J.; Ramaekers, F.C.; Schutte, B.; Reutelingsperger, C.P. Annexin V-affinity assay: A review on an apoptosis detection system based on phosphatidylserine exposure. Cytometry 1998, 31, 1–9. [Google Scholar] [CrossRef]
- Deng, Z.; Gao, P.; Yu, L.; Ma, B.; You, Y.; Chan, L.; Mei, C.; Chen, T. Ruthenium complexes with phenylterpyridine derivatives target cell membrane and trigger death receptors-mediated apoptosis in cancer cells. Biomaterials 2017, 129, 111–126. [Google Scholar] [CrossRef] [PubMed]
- Wan, D.; Lai, S.H.; Zeng, C.C.; Zhang, C.; Tang, B.; Liu, Y.J. Ruthenium(II) polypyridyl complexes: Synthesis, characterization and anticancer activity studies on BEL-7402 cells. J. Inorg. Biochem. 2017, 173, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Santos, L.S.; Silva, V.R.; Menezes, L.R.A.; Soares, M.B.P.; Costa, E.V.; Bezerra, D.P. Xylopine induces oxidative stress and causes G2/M Phase arrest, triggering caspase-mediated apoptosis by p53-independent pathway in HCT116 cells. Oxid. Med. Cell. Longev. 2017, 2017, 7126872. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.A.; Gogal, R.M., Jr.; Walsh, J.E. A new rapid and simple non-radioactive assay to monitor and determine the proliferation of lymphocytes: An alternative to [3H]thymidine incorporation assay. J. Immunol. Methods 1994, 170, 211–224. [Google Scholar] [CrossRef]
- Nicoletti, I.; Migliorati, G.; Pagliacci, M.C.; Grignani, F.; Riccardi, C. A rapid and simple method for measuring thymocyte apoptosis by propidium iodide staining and flow cytometry. J. Immunol. Methods 1991, 139, 271–279. [Google Scholar] [CrossRef]
- Sureda, F.X.; Escubedo, E.; Gabriel, C.; Comas, J.; Camarasa, J.; Camins, A. Mitochondrial membrane potential measurement in rat cerebellar neurons by flow cytometry. Cytometry 1997, 28, 74–80. [Google Scholar] [CrossRef]
- LeBel, C.P.; Ischiropoulos, H.; Bondy, S.C. Evaluation of the probe 2′,7′-dichlorofluorescin as an indicator of reactive oxygen species formation and oxidative stress. Chem. Res. Toxicol. 1992, 5, 227–231. [Google Scholar] [CrossRef] [PubMed]








| Cells | Histological Type | IC50 in µM | ||
|---|---|---|---|---|
| DOX | OXA | CRT | ||
| Cancer cells | ||||
| HL-60 | Human promyelocytic leukemia | 0.3 | 0.6 | 1.4 |
| 0.3–0.4 | 0.1–0.8 | 0.7–2.8 | ||
| K562 | Human chronic myelogenous leukemia | 0.3 | 1.0 | 1.3 |
| 0.2–0.5 | 0.1–1.3 | 0.9–2.0 | ||
| HCT116 | Human colon carcinoma | 0.1 | 4.1 | 1.6 |
| 0.1–0.2 | 2.7–6.4 | 1.2–2.2 | ||
| MCF7 | Human breast carcinoma | 1.1 | 5.7 | 11.1 |
| 0.3–3.5 | 3.3–9.4 | 7.9–15.5 | ||
| HepG2 | Human hepatocellular carcinoma | 0.1 | 2.2 | 2.8 |
| 0.1–0.2 | 1.3–3.8 | 2.3–3.3 | ||
| HSC3 | Human oral squamous cell carcinoma | 0.3 | 3.1 | 1.1 |
| 0.2–0.4 | 1.6–5.3 | 0.8–1.5 | ||
| SCC9 | Human oral squamous cell carcinoma | 0.5 | N.d. | 2.4 |
| 0.4–0.7 | 1.7–3.5 | |||
| B16-F10 | Mouse melanoma | 0.1 | 2.2 | 1.4 |
| 0.1–0.2 | 1.2–4.1 | 1.2–1.8 | ||
| Non-cancer cells | ||||
| MRC5 | Human lung fibroblast | 1.5 | 1.3 | 11.5 |
| 1.2–2.0 | 1.0–2.2 | 10.3–12.9 | ||
| PBMC | Human peripheral blood mononuclear cells | 5.1 | 9.4 | 1.7 |
| 3.2–8.2 | 6.5–11.4 | 1.4–2.0 | ||
| Cancer Cells | Non-Cancer Cells | |||||
|---|---|---|---|---|---|---|
| MRC5 | PBMC | |||||
| DOX | OXA | CRT | DOX | OXA | CRT | |
| HL-60 | 5 | 2.2 | 8.2 | 17 | 15.7 | 1.2 |
| K-562 | 5 | 1.3 | 8.8 | 17 | 9.4 | 1.3 |
| HCT116 | 15 | 0.3 | 7.2 | 51 | 2.3 | 1.1 |
| MCF7 | 1.4 | 0.2 | 1 | 4.6 | 1.7 | 0.2 |
| HepG2 | 15 | 0.6 | 4.1 | 51 | 4.3 | 0.6 |
| HSC-3 | 5 | 0.4 | 10.5 | 17 | 3.0 | 1.6 |
| SCC-9 | 3 | N.d. | 4.8 | 10.2 | N.d. | 0.7 |
| B16-F10 | 15 | 0.6 | 8.2 | 51 | 4.3 | 1.2 |
| Treatment | Concentration (µM) | DNA Content (%) | |||
|---|---|---|---|---|---|
| Sub-G0/G1 | G0/G1 | S | G2/M | ||
| 24 h of incubation | |||||
| CTL | - | 11.5 ± 1.4 | 50.7 ± 5.5 | 15.0 ± 2.5 | 16.3 ± 0.7 |
| DOX | 2 | 58.6 ± 2.5 * | 19.9 ± 2.5 * | 6.6 ± 1.6 * | 8.9 ± 1.5 * |
| OXA | 2.5 | 21.9 ± 3.3 * | 31.4 ± 3.0 * | 11.6 ± 2.3 | 26.0 ± 3.6 * |
| CRT | 1 | 19.4 ± 3.9 * | 30.5 ± 4.8 * | 11.5 ± 1.7 | 18.5 ± 0.7 |
| 2 | 30.1 ± 7.9 * | 38.7 ± 2.2 * | 7.8 ± 1.5 * | 17.9 ± 4.4 | |
| 4 | 36.2 ± 9.5 * | 36.3 ± 3.1 * | 10.4 ± 2.4 | 13.2 ± 4.1 | |
| 48 h of incubation | |||||
| CTL | - | 7.5 ± 1.0 | 58.5 ± 3.1 | 14.1 ± 1.3 | 17.0 ± 1.6 |
| DOX | 2 | 65.4 ± 2.8 * | 21.9 ± 3.0 * | 6.5 ± 0.6 * | 3.8 ± 0.9 * |
| OXA | 2.5 | 18.4 ± 4.4 * | 42.9 ± 2.8 * | 14.2 ± 1.2 | 19.1 ± 3.3 |
| CRT | 1 | 12.5 ± 3.5 * | 41.2 ± 9.6 * | 11.2 ± 2.4 | 10.8 ± 2.5 |
| 2 | 26.7 ± 7.0 * | 42.6 ± 4.9 * | 10.5 ± 0.8 * | 14.4 ± 2.5 | |
| 4 | 58.2 ± 8.8 * | 28.4 ± 5.9 * | 4.4 ± 1.2 * | 5.5 ± 2.6 * | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Souza Oliveira, M.; De Santana, Á.A.D.; Correa, R.S.; Soares, M.B.P.; Batista, A.A.; Bezerra, D.P. Ru(II)-Thymine Complex Causes Cell Growth Inhibition and Induction of Caspase-Mediated Apoptosis in Human Promyelocytic Leukemia HL-60 Cells. Int. J. Mol. Sci. 2018, 19, 1609. https://doi.org/10.3390/ijms19061609
De Souza Oliveira M, De Santana ÁAD, Correa RS, Soares MBP, Batista AA, Bezerra DP. Ru(II)-Thymine Complex Causes Cell Growth Inhibition and Induction of Caspase-Mediated Apoptosis in Human Promyelocytic Leukemia HL-60 Cells. International Journal of Molecular Sciences. 2018; 19(6):1609. https://doi.org/10.3390/ijms19061609
Chicago/Turabian StyleDe Souza Oliveira, Maiara, Ádila Angélica Dantas De Santana, Rodrigo S. Correa, Milena Botelho Pereira Soares, Alzir Azevedo Batista, and Daniel Pereira Bezerra. 2018. "Ru(II)-Thymine Complex Causes Cell Growth Inhibition and Induction of Caspase-Mediated Apoptosis in Human Promyelocytic Leukemia HL-60 Cells" International Journal of Molecular Sciences 19, no. 6: 1609. https://doi.org/10.3390/ijms19061609