Molecular Analysis of Sensory Axon Branching Unraveled a cGMP-Dependent Signaling Cascade
Abstract
:1. Introduction: Axonal Pathfinding and Branching—Fundamental Processes to Establish Neuronal Circuits
2. cGMP Signaling and Growth Cone Steering—Initial Cell Culture Studies
3. Branching of Sensory Axons within the Spinal Cord—A Versatile System to Characterize Intracellular Signaling Implicated in Axon Branching
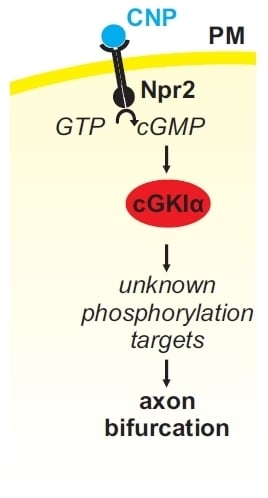
4. A cGMP Signaling Cascade Regulates Axon Bifurcation but Not Collateral Formation or Terminal Branching
5. The CNP/Npr2/cGKI Signaling Cascade Induces Bifurcation of Axons from Three Types of Neurons: DRG, Cranial Sensory Ganglia (CSG), and Mesencephalic Trigeminal Neurons (MTN)
6. The Role of Phosphodiesterase 2A and the Scavenger Receptor Npr3 in Sensory Axon Bifurcation
7. Behavioral Consequences of the Absence of Sensory Axon Bifurcation: Nociception Is Impaired, Whereas Motor Balance and Coordination Is Normal
8. Compensatory Mechanisms Alter the Spatial Extension of Receptive Fields in the Spinal Cord in the Absence of Sensory Axon Bifurcation
9. CNP/Npr2 Signaling in Human Diseases
Author Contributions
Acknowledgments
Conflicts of Interest
Abbreviations
| AMDM | Acromesomelic dysplasia, Maroteaux type |
| ANP | Atrial natriuretic peptide |
| BNP | Brain natriuretic peptide |
| C | Caudal |
| cGKI | cGMP-dependent kinase I |
| CNP | C-type natriuretic peptide |
| CSG | Cranial sensory ganglia |
| D | Dorsal |
| DEA/NO | 2-(N,N-dethylamino)-diazenolate-2-oxide dethylammonium salt |
| DREZ | Dorsal root entry zone |
| DRG | Dorsal root ganglia |
| DSC | Dorsal spinal cord |
| Fb | Forebrain |
| gV | Trigeminal ganglion |
| Hb | Hindbrain |
| MAP7 | Microtubule-associated protein 7 |
| Mb | Midbrain |
| MTN | Mesencephalic trigeminal neuron |
| NO | Nitric oxide |
| NO-GC | Nitric oxide guanylyl cyclase |
| Npr2 | Natriuretic peptide receptor 2 |
| Npr3 | Natriuretic peptide receptor 3 |
| PDE | Phosphodiesterase |
| PM | Plasma membrane |
| R | Rostral |
| SC | Spinal cord |
| V | Ventral |
| Vmo | Trigeminal motor nucleus |
References
- Tojima, T.; Hines, J.H.; Henley, J.R.; Kamiguchi, H. Second messengers and membrane trafficking direct and organize growth cone steering. Nat. Rev. Neurosci. 2011, 12, 191–203. [Google Scholar] [CrossRef] [PubMed]
- Dickson, B.J. Molecular mechanisms of axon guidance. Science 2002, 298, 1959–1964. [Google Scholar] [CrossRef] [PubMed]
- Schnorrer, F.; Dickson, B.J. Axon guidance: Morphogens show the way. Curr. Biol. 2004, 14, R19–R21. [Google Scholar] [CrossRef] [PubMed]
- Seiradake, E.; Jones, E.Y.; Klein, R. Structural Perspectives on Axon Guidance. Annu. Rev. Cell Dev. Biol. 2016, 32, 577–608. [Google Scholar] [CrossRef] [PubMed]
- Petrovic, M.; Schmucker, D. Axonal wiring in neural development: Target-independent mechanisms help to establish precision and complexity. Bioessays 2015, 37, 996–1004. [Google Scholar] [CrossRef] [PubMed]
- Nugent, A.A.; Kolpak, A.L.; Engle, E.C. Human disorders of axon guidance. Curr. Opin. Neurobiol. 2012, 22, 837–843. [Google Scholar] [CrossRef] [PubMed]
- Chedotal, A. Development and plasticity of commissural circuits: From locomotion to brain repair. Trends Neurosci. 2014, 37, 551–562. [Google Scholar] [CrossRef] [PubMed]
- Engle, E.C. Human genetic disorders of axon guidance. Cold Spring Harb. Perspect. Biol. 2010, 2, a001784. [Google Scholar] [CrossRef] [PubMed]
- Kalil, K.; Li, L.; Hutchins, B.I. Signaling mechanisms in cortical axon growth, guidance, and branching. Front Neuroanat. 2011, 5, 62. [Google Scholar] [CrossRef] [PubMed]
- Kalil, K.; Dent, E.W. Branch management: Mechanisms of axon branching in the developing vertebrate CNS. Nat. Rev. Neurosci. 2014, 15, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Averaimo, S.; Nicol, X. Intermingled cAMP, cGMP and calcium spatiotemporal dynamics in developing neuronal circuits. Front. Cell. Neurosci. 2014, 8, 376. [Google Scholar] [CrossRef] [PubMed]
- Winkle, C.C.; Taylor, K.L.; Dent, E.W.; Gallo, G.; Greif, K.F.; Gupton, S.L. Beyond the cytoskeleton: The emerging role of organelles and membrane remodeling in the regulation of axon collateral branches. Dev. Neurobiol. 2016, 76, 1293–1307. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, F.; Feil, R.; Kleppisch, T.; Schlossmann, J. Function of cGMP-dependent protein kinases as revealed by gene deletion. Physiol. Rev. 2006, 86, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Kemp-Harper, B.; Feil, R. Meeting report: CGMP matters. Sci. Signal. 2008, 1, e12. [Google Scholar] [CrossRef] [PubMed]
- Song, H.J.; Poo, M.M. Signal transduction underlying growth cone guidance by diffusible factors. Curr. Opin. Neurobiol. 1999, 9, 355–363. [Google Scholar] [CrossRef]
- Song, H.; Ming, G.; He, Z.; Lehmann, M.; McKerracher, L.; Tessier-Lavigne, M.; Poo, M. Conversion of neuronal growth cone responses from repulsion to attraction by cyclic nucleotides. Science 1998, 281, 1515–1518. [Google Scholar] [CrossRef] [PubMed]
- Nishiyama, M.; von Schimmelmann, M.J.; Togashi, K.; Findley, W.M.; Hong, K. Membrane potential shifts caused by diffusible guidance signals direct growth-cone turning. Nat. Neurosci. 2008, 11, 762–771. [Google Scholar] [CrossRef] [PubMed]
- Henley, J.R.; Huang, K.H.; Wang, D.; Poo, M.M. Calcium mediates bidirectional growth cone turning induced by myelin-associated glycoprotein. Neuron 2004, 44, 909–916. [Google Scholar] [CrossRef] [PubMed]
- Henley, J.; Poo, M.M. Guiding neuronal growth cones using Ca2+ signals. Trends Cell Biol. 2004, 14, 320–330. [Google Scholar] [CrossRef] [PubMed]
- Hopker, V.H.; Shewan, D.; Tessier-Lavigne, M.; Poo, M.; Holt, C. Growth-cone attraction to netrin-1 is converted to repulsion by laminin-1. Nature 1999, 401, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Nishiyama, M.; Hoshino, A.; Tsai, L.; Henley, J.R.; Goshima, Y.; Tessier-Lavigne, M.; Poo, M.M.; Hong, K. Cyclic AMP/GMP-dependent modulation of Ca2+ channels sets the polarity of nerve growth-cone turning. Nature 2003, 423, 990–995. [Google Scholar] [CrossRef] [PubMed]
- Ooashi, N.; Futatsugi, A.; Yoshihara, F.; Mikoshiba, K.; Kamiguchi, H. Cell adhesion molecules regulate Ca2+-mediated steering of growth cones via cyclic AMP and ryanodine receptor type 3. J. Cell Biol. 2005, 170, 1159–1167. [Google Scholar] [CrossRef] [PubMed]
- Tojima, T.; Itofusa, R.; Kamiguchi, H. The nitric oxide-cGMP pathway controls the directional polarity of growth cone guidance via modulating cytosolic Ca2+ signals. J. Neurosci. 2009, 29, 7886–7897. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, H.; Fukuda, T.; Tojima, T.; Nikolaev, V.O.; Kamiguchi, H. Cyclic Nucleotide Control of Microtubule Dynamics for Axon Guidance. J. Neurosci. 2016, 36, 5636–5649. [Google Scholar] [CrossRef] [PubMed]
- Tojima, T.; Akiyama, H.; Itofusa, R.; Li, Y.; Katayama, H.; Miyawaki, A.; Kamiguchi, H. Attractive axon guidance involves asymmetric membrane transport and exocytosis in the growth cone. Nat. Neurosci. 2007, 10, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Tojima, T.; Itofusa, R.; Kamiguchi, H. Steering neuronal growth cones by shifting the imbalance between exocytosis and endocytosis. J. Neurosci. 2014, 34, 7165–7178. [Google Scholar] [CrossRef] [PubMed]
- Keleman, K.; Ribeiro, C.; Dickson, B.J. Comm function in commissural axon guidance: Cell-autonomous sorting of Robo in vivo. Nat. Neurosci. 2005, 8, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Philipp, M.; Niederkofler, V.; Debrunner, M.; Alther, T.; Kunz, B.; Stoeckli, E.T. RabGDI controls axonal midline crossing by regulating Robo1 surface expression. Neural Dev. 2012, 7, 36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cogen, J.; Cohen-Cory, S. Nitric oxide modulates retinal ganglion cell axon arbor remodeling in vivo. J. Neurobiol. 2000, 45, 120–133. [Google Scholar] [CrossRef]
- Polleux, F.; Morrow, T.; Ghosh, A. Semaphorin 3A is a chemoattractant for cortical apical dendrites. Nature 2000, 404, 567–573. [Google Scholar] [CrossRef] [PubMed]
- Xiong, G.; Mojsilovic-Petrovic, J.; Perez, C.A.; Kalb, R.G. Embryonic motor neuron dendrite growth is stunted by inhibition of nitric oxide-dependent activation of soluble guanylyl cyclase and protein kinase G. Eur. J. Neurosci. 2007, 25, 1987–1997. [Google Scholar] [CrossRef] [PubMed]
- Carrel, D.; Du, Y.; Komlos, D.; Hadzimichalis, N.M.; Kwon, M.; Wang, B.; Brzustowicz, L.M.; Firestein, B.L. NOS1AP regulates dendrite patterning of hippocampal neurons through a carboxypeptidase E-mediated pathway. J. Neurosci. 2009, 29, 8248–8258. [Google Scholar] [CrossRef] [PubMed]
- Helmstaedter, M.; Sakmann, B.; Feldmeyer, D. Neuronal correlates of local, lateral, and translaminar inhibition with reference to cortical columns. Cereb. Cortex 2009, 19, 926–937. [Google Scholar] [CrossRef] [PubMed]
- Tamas, G.; Buhl, E.H.; Somogyi, P. Massive autaptic self-innervation of GABAergic neurons in cat visual cortex. J. Neurosci. 1997, 17, 6352–6364. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.G. Organization in the Spinal Cord; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 1981. [Google Scholar]
- Ha, H. Axonal bifurcation in the dorsal root ganglion of the cat: A light and electron microscopic study. J. Comp. Neurol. 1970, 140, 227–240. [Google Scholar] [CrossRef] [PubMed]
- Ozaki, S.; Snider, W.D. Initial trajectories of sensory axons toward laminar targets in the developing mouse spinal cord. J. Comp. Neurol. 1997, 380, 215–229. [Google Scholar] [CrossRef]
- Mirnics, K.; Koerber, H.R. Prenatal development of rat primary afferent fibers: II. Central projections. J. Comp. Neurol. 1995, 355, 601–614. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, H.; Rathjen, F.G. Signalling mechanisms regulating axonal branching in vivo. Bioessays 2010, 32, 977–985. [Google Scholar] [CrossRef] [PubMed]
- Gibson, D.A.; Ma, L. Developmental regulation of axon branching in the vertebrate nervous system. Development 2011, 138, 183–195. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, H.; Rathjen, F.G. DiI-labeling of DRG neurons to study axonal branching in a whole mount preparation of mouse embryonic spinal cord. J. Vis. Exp. 2011, e3667. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, H.; Ter-Avetisyan, G.; Rathjen, F.G. A genetic strategy for the analysis of individual axon morphologies in cGMP signalling mutant mice. Methods Mol. Biol. 2013, 1020, 193–204. [Google Scholar] [PubMed]
- Ter-Avetisyan, G.; Rathjen, F.G.; Schmidt, H. Bifurcation of axons from cranial sensory neurons is disabled in the absence of Npr2-induced cGMP signaling. J. Neurosci. 2014, 34, 737–747. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, H.; Werner, M.; Heppenstall, P.A.; Henning, M.; More, M.I.; Kuhbandner, S.; Lewin, G.R.; Hofmann, F.; Feil, R.; Rathjen, F.G. cGMP-mediated signaling via cGKIalpha is required for the guidance and connectivity of sensory axons. J. Cell Biol. 2002, 159, 489–498. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, H.; Stonkute, A.; Juttner, R.; Schaffer, S.; Buttgereit, J.; Feil, R.; Hofmann, F.; Rathjen, F.G. The receptor guanylyl cyclase Npr2 is essential for sensory axon bifurcation within the spinal cord. J. Cell Biol. 2007, 179, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, H.; Stonkute, A.; Juttner, R.; Koesling, D.; Friebe, A.; Rathjen, F.G. C-type natriuretic peptide (CNP) is a bifurcation factor for sensory neurons. Proc. Natl. Acad. Sci. USA 2009, 106, 16847–16852. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Wang, Z.; Gu, Y.; Feil, R.; Hofmann, F.; Ma, L. Regulate axon branching by the cyclic GMP pathway via inhibition of glycogen synthase kinase 3 in dorsal root ganglion sensory neurons. J. Neurosci. 2009, 29, 1350–1360. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Ma, L. Regulation of axonal development by natriuretic peptide hormones. Proc. Natl. Acad. Sci. USA 2009, 106, 18016–18021. [Google Scholar] [CrossRef] [PubMed]
- Lallemend, F.; Ernfors, P. Molecular interactions underlying the specification of sensory neurons. Trends Neurosci. 2012, 35, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Xia, C.; Nguyen, M.; Garrison, A.K.; Zhao, Z.; Wang, Z.; Sutherland, C.; Ma, L. CNP/cGMP signaling regulates axon branching and growth by modulating microtubule polymerization. Dev. Neurobiol. 2013, 73, 673–687. [Google Scholar] [CrossRef] [PubMed]
- Koesling, D.; Russwurm, M.; Mergia, E.; Mullershausen, F.; Friebe, A. Nitric oxide-sensitive guanylyl cyclase: Structure and regulation. Neurochem. Int. 2004, 45, 813–819. [Google Scholar] [CrossRef] [PubMed]
- Friebe, A.; Mergia, E.; Dangel, O.; Lange, A.; Koesling, D. Fatal gastrointestinal obstruction and hypertension in mice lacking nitric oxide-sensitive guanylyl cyclase. Proc. Natl. Acad. Sci. USA 2007, 104, 7699–7704. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, H.; Peters, S.; Frank, K.; Wen, L.; Feil, R.; Rathjen, F.G. Dorsal root ganglion axon bifurcation tolerates increased cyclic GMP levels: The role of phosphodiesterase 2A and scavenger receptor Npr3. Eur. J. Neurosci. 2016, 44, 2991–3000. [Google Scholar] [CrossRef] [PubMed]
- Tymanskyj, S.R.; Yang, B.; Falnikar, A.; Lepore, A.C.; Ma, L. MAP7 Regulates Axon Collateral Branch Development in Dorsal Root Ganglion Neurons. J. Neurosci. 2017, 37, 1648–1661. [Google Scholar] [CrossRef] [PubMed]
- Lleras-Forero, L.; Streit, A. Development of the sensory nervous system in the vertebrate head: The importance of being on time. Curr. Opin. Genet. Dev. 2012, 22, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Ladher, R.K.; O’Neill, P.; Begbie, J. From shared lineage to distinct functions: The development of the inner ear and epibranchial placodes. Development 2010, 137, 1777–1785. [Google Scholar] [CrossRef] [PubMed]
- Ratcliffe, E.M.; Farrar, N.R.; Fox, E.A. Development of the vagal innervation of the gut: Steering the wandering nerve. Neurogastroenterol. Motil. 2011, 23, 898–911. [Google Scholar] [CrossRef] [PubMed]
- Chedotal, A.; Pourquie, O.; Sotelo, C. Initial tract formation in the brain of the chick embryo: Selective expression of the BEN/SC1/DM-GRASP cell adhesion molecule. Eur. J. Neurosci. 1995, 7, 198–212. [Google Scholar] [CrossRef] [PubMed]
- Mastick, G.S.; Easter, S.S., Jr. Initial organization of neurons and tracts in the embryonic mouse fore- and midbrain. Dev. Biol. 1996, 173, 79–94. [Google Scholar] [CrossRef] [PubMed]
- Molle, K.D.; Chedotal, A.; Rao, Y.; Lumsden, A.; Wizenmann, A. Local inhibition guides the trajectory of early longitudinal tracts in the developing chick brain. Mech. Dev. 2004, 121, 143–156. [Google Scholar] [CrossRef] [PubMed]
- Ware, M.; Schubert, F.R. Development of the early axon scaffold in the rostral brain of the chick embryo. J. Anat. 2011, 219, 203–216. [Google Scholar] [CrossRef] [PubMed]
- Shigenaga, Y.; Mitsuhiro, Y.; Yoshida, A.; Cao, C.Q.; Tsuru, H. Morphology of single mesencephalic trigeminal neurons innervating masseter muscle of the cat. Brain Res. 1988, 445, 392–399. [Google Scholar] [CrossRef]
- Luo, P.F.; Wang, B.R.; Peng, Z.Z.; Li, J.S. Morphological characteristics and terminating patterns of masseteric neurons of the mesencephalic trigeminal nucleus in the rat: An intracellular horseradish peroxidase labeling study. J. Comp. Neurol. 1991, 303, 286–299. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, A.; Moritani, M.; Nagase, Y.; Bae, Y.C. Projection and synaptic connectivity of trigeminal mesencephalic nucleus neurons controlling jaw reflexes. J. Oral Sci. 2017, 59, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Widmer, C.G.; Morris-Wiman, J.A.; Calhoun, J.C. Development of trigeminal mesencephalic and motor nuclei in relation to masseter muscle innervation in mice. Brain Res. Dev. Brain Res. 1998, 108, 1–11. [Google Scholar] [CrossRef]
- Turman, J.E., Jr. The development of mastication in rodents: From neurons to behaviors. Arch. Oral Biol. 2007, 52, 313–316. [Google Scholar] [CrossRef] [PubMed]
- Hunter, E.; Begbie, J.; Mason, I.; Graham, A. Early development of the mesencephalic trigeminal nucleus. Dev. Dyn. 2001, 222, 484–493. [Google Scholar] [CrossRef] [PubMed]
- Dessem, D.; Taylor, A. Morphology of jaw-muscle spindle afferents in the rat. J. Comp. Neurol. 1989, 282, 389–403. [Google Scholar] [CrossRef] [PubMed]
- Lazarov, N.E. Neurobiology of orofacial proprioception. Brain Res. Rev. 2007, 56, 362–383. [Google Scholar] [CrossRef] [PubMed]
- Ter-Avetisyan, G.; Dumoulin, A.; Herrel, A.; Schmidt, H.; Strump, J.; Afzal, S.; Rathjen, F.G. Loss of axon bifurcation in mesencephalic trigeminal neurons impairs the maximal biting force in Npr2-deficient mice. Unpublished work. Front. Cell. Neurosci. 2018; In revision. [Google Scholar]
- Xu, Y.; Zhang, H.T.; O’Donnell, J.M. Phosphodiesterases in the central nervous system: Implications in mood and cognitive disorders. Handb. Exp. Pharmacol. 2011, 447–485. [Google Scholar] [CrossRef]
- Maurice, D.H.; Ke, H.; Ahmad, F.; Wang, Y.; Chung, J.; Manganiello, V.C. Advances in targeting cyclic nucleotide phosphodiesterases. Nat. Rev. Drug Discov. 2014, 13, 290–314. [Google Scholar] [CrossRef] [PubMed]
- Jaubert, J.; Jaubert, F.; Martin, N.; Washburn, L.L.; Lee, B.K.; Eicher, E.M.; Guenet, J.L. Three new allelic mouse mutations that cause skeletal overgrowth involve the natriuretic peptide receptor C gene (Npr3). Proc. Natl. Acad. Sci. USA 1999, 96, 10278–10283. [Google Scholar] [CrossRef] [PubMed]
- Matsukawa, N.; Grzesik, W.J.; Takahashi, N.; Pandey, K.N.; Pang, S.; Yamauchi, M.; Smithies, O. The natriuretic peptide clearance receptor locally modulates the physiological effects of the natriuretic peptide system. Proc. Natl. Acad. Sci. USA 1999, 96, 7403–7408. [Google Scholar] [CrossRef] [PubMed]
- Schouenborg, J. Learning in sensorimotor circuits. Curr. Opin. Neurobiol. 2004, 14, 693–697. [Google Scholar] [CrossRef] [PubMed]
- Granmo, M.; Petersson, P.; Schouenborg, J. Action-based body maps in the spinal cord emerge from a transitory floating organization. J. Neurosci. 2008, 28, 5494–5503. [Google Scholar] [CrossRef] [PubMed]
- Troster, P.; Haseleu, J.; Petersen, J.; Drees, O.; Schmidtko, A.; Schwaller, F.; Lewin, G.R.; Ter-Avetisyan, G.; Winter, Y.; Peters, S.; et al. The Absence of Sensory Axon Bifurcation Affects Nociception and Termination Fields of Afferents in the Spinal Cord. Front Mol. Neurosci. 2018, 11, 19. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.C.; Cao, X.J.; Wright, S.; Ma, L.; Oertel, D.; Goodrich, L.V. Mutation of Npr2 leads to blurred tonotopic organization of central auditory circuits in mice. PLoS. Genet. 2014, 10, e1004823. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bartels, C.F.; Bukulmez, H.; Padayatti, P.; Rhee, D.K.; Ravenswaaij-Arts, C.; Pauli, R.M.; Mundlos, S.; Chitayat, D.; Shih, L.Y.; Al Gazali, L.I.; et al. Mutations in the transmembrane natriuretic peptide receptor NPR-B impair skeletal growth and cause acromesomelic dysplasia, type Maroteaux. Am. J. Hum. Genet. 2004, 75, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Potter, L.R. Guanylyl cyclase structure, function and regulation. Cell Signal. 2011, 23, 1921–1926. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, M. Molecular Physiology of Membrane Guanylyl Cyclase Receptors. Physiol. Rev. 2016, 96, 751–804. [Google Scholar] [CrossRef] [PubMed]
- Bocciardi, R.; Giorda, R.; Buttgereit, J.; Gimelli, S.; Divizia, M.T.; Beri, S.; Garofalo, S.; Tavella, S.; Lerone, M.; Zuffardi, O.; et al. Overexpression of the C-type natriuretic peptide (CNP) is associated with overgrowth and bone anomalies in an individual with balanced t(2;7) translocation. Hum. Mutat. 2007, 28, 724–731. [Google Scholar] [CrossRef] [PubMed]
- Moncla, A.; Missirian, C.; Cacciagli, P.; Balzamo, E.; Legeai-Mallet, L.; Jouve, J.L.; Chabrol, B.; Le, M.M.; Plessis, G.; Villard, L.; et al. A cluster of translocation breakpoints in 2q37 is associated with overexpression of NPPC in patients with a similar overgrowth phenotype. Hum. Mutat. 2007, 28, 1183–1188. [Google Scholar] [CrossRef] [PubMed]
- Chusho, H.; Tamura, N.; Ogawa, Y.; Yasoda, A.; Suda, M.; Miyazawa, T.; Nakamura, K.; Nakao, K.; Kurihara, T.; Komatsu, Y.; et al. Dwarfism and early death in mice lacking C-type natriuretic peptide. Proc. Natl. Acad. Sci. USA 2001, 98, 4016–4021. [Google Scholar] [CrossRef] [PubMed]
- Tamura, N.; Doolittle, L.K.; Hammer, R.E.; Shelton, J.M.; Richardson, J.A.; Garbers, D.L. Critical roles of the guanylyl cyclase B receptor in endochondral ossification and development of female reproductive organs. Proc. Natl. Acad. Sci. USA 2004, 101, 17300–17305. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, T.; Kunieda, T. A loss-of-function mutation in natriuretic peptide receptor 2 (Npr2) gene is responsible for disproportionate dwarfism in cn/cn mouse. J. Biol. Chem. 2005, 280, 14288–14292. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Yan, J.; Jiao, F.; Yang, H.; Donahue, L.R.; Li, X.; Roe, B.A.; Stuart, J.; Gu, W. A single nucleotide mutation in Nppc is associated with a long bone abnormality in lbab mice. BMC. Genet. 2007, 8, 16. [Google Scholar] [CrossRef] [PubMed]
- Nakao, K.; Osawa, K.; Yasoda, A.; Yamanaka, S.; Fujii, T.; Kondo, E.; Koyama, N.; Kanamoto, N.; Miura, M.; Kuwahara, K.; et al. The Local CNP/GC-B system in growth plate is responsible for physiological endochondral bone growth. Sci. Rep. 2015, 5, 10554. [Google Scholar] [CrossRef] [PubMed] [Green Version]





© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dumoulin, A.; Ter-Avetisyan, G.; Schmidt, H.; Rathjen, F.G. Molecular Analysis of Sensory Axon Branching Unraveled a cGMP-Dependent Signaling Cascade. Int. J. Mol. Sci. 2018, 19, 1266. https://doi.org/10.3390/ijms19051266
Dumoulin A, Ter-Avetisyan G, Schmidt H, Rathjen FG. Molecular Analysis of Sensory Axon Branching Unraveled a cGMP-Dependent Signaling Cascade. International Journal of Molecular Sciences. 2018; 19(5):1266. https://doi.org/10.3390/ijms19051266
Chicago/Turabian StyleDumoulin, Alexandre, Gohar Ter-Avetisyan, Hannes Schmidt, and Fritz G. Rathjen. 2018. "Molecular Analysis of Sensory Axon Branching Unraveled a cGMP-Dependent Signaling Cascade" International Journal of Molecular Sciences 19, no. 5: 1266. https://doi.org/10.3390/ijms19051266