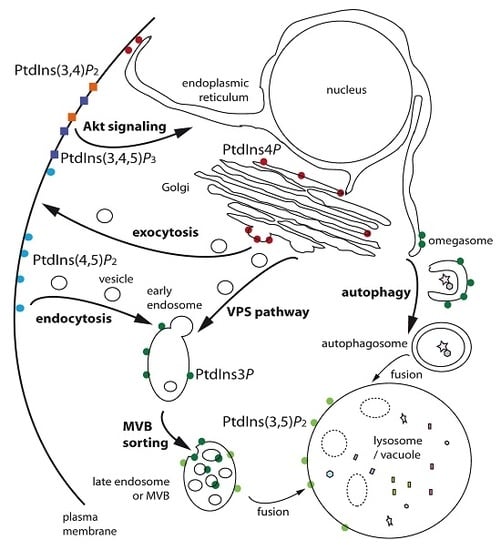
Phosphoinositides, Major Actors in Membrane Trafficking and Lipid Signaling Pathways
Abstract
:1. Phosphoinositides in Cellular Membranes
1.1. Lipids, Major Membrane Components
1.2. Phosphoinositides, Lipid Signaling Molecules
1.3. Phosphatidylinositol, the Precursor of Phosphoinositides
2. PtdIns4P a Key Trafficking Effector for Phospholipids and Sterols
2.1. PtdIns4P Synthesis
2.2. Physiological Role of PtdIns4P
3. Phosphatidylinositol 3-Phosphate (PtdIns3P) an Endosomal Lipid Essential in Membrane Trafficking and Autophagy
3.1. PtdIns3P Synthesis
- -
- Two members of the class I phosphoinositide 3-kinases (PI3KC), which phosphorylate predominantly PtdIns(4,5)P2 to generate PtdIns(3,4,5)P3
- -
- Three members of the class II phosphoinositide 3-kinases (PIK3C2), which predominantly phosphorylate PtdIns4P to generate PtdIns(3,4)P2
- -
- One member of the class III phosphoinositide 3-kinase (homologous to the yeast Vps34 lipid kinase). As Vps34, the human PIK3C3 is specific for PtdIns and is consequently most probably the biggest source of cellular PtdIns3P. The regulatory subunit of VPS34/PIK3C3 is the protein p150/PIK3R4, the homologue of the yeast Vps15 [42]. A phylogenetic study suggests the co-evolution of VPS34/PIK3C3 and its regulatory subunit VPS15/PIK3R4 in most eukaryotes, from yeast to human and plants and in many protists, such as amoeba [43]. However, a recent study shows that in human cells, VPS15 has an additional function in trafficking from Golgi to primary cilia, independent from its association with VPS34, and that a missense mutation in the VPS15 gene is responsible for ciliopathy [44].
3.2. Physiological Role of PtdIns3P
4. PtdIns5P, an Underappreciated Phosphoinositide
4.1. PtdIns5P Synthesis
4.2. Physiological Role of PtdIns5P
5. PtdIns(4,5)P2, a Phosphoinositide Involved in Actin Dynamics and in Endocytosis
5.1. PtdIns(4,5)P2 Synthesis
5.2. Physiological Role of PtdIns(4,5)P2
6. PtdIns(3,5)P2, a Regulator of Endosome-Lysosome Trafficking
6.1. PtdIns(3,5)P2 Synthesis
6.2. Physiological Role of PtdIns(3,5)P2
7. PtdIns(3,4)P2, a Lipid Secondary Messenger
7.1. PtdIns(3,4)P2 Synthesis
7.2. Physiological Role of PtdIns(3,4)P2
8. PtdIns(3,4,5)P3, a Key Effector of the PI3K/Akt Signaling Pathway
8.1. PtdIns(3,4,5)P3 Synthesis
8.2. Physiological Role of PtdIns(3,4,5)P3
9. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Spector, A.A.; Yorek, M.A. Membrane lipid composition and cellular function. J. Lipid Res. 1985, 26, 1015–1035. [Google Scholar] [PubMed]
- Zinser, E.; Sperka-Gottlieb, C.D.; Fasch, E.V.; Kohlwein, S.D.; Paltauf, F.; Daum, G. Phospholipid synthesis and lipid composition of subcellular membranes in the unicellular eukaryote saccharomyces cerevisiae. J. Bacteriol. 1991, 173, 2026–2034. [Google Scholar] [CrossRef] [PubMed]
- Kornberg, R.D.; McConnell, H.M. Lateral diffusion of phospholipids in a vesicle membrane. Proc. Natl. Acad. Sci. USA 1971, 68, 2564–2568. [Google Scholar] [CrossRef] [PubMed]
- Nakano, M.; Fukuda, M.; Kudo, T.; Matsuzaki, N.; Azuma, T.; Sekine, K.; Endo, H.; Handa, T. Flip-flop of phospholipids in vesicles: Kinetic analysis with time-resolved small-angle neutron scattering. J. Phys. Chem. B 2009, 113, 6745–6748. [Google Scholar] [CrossRef] [PubMed]
- Michell, R.H.; Heath, V.L.; Lemmon, M.A.; Dove, S.K. Phosphatidylinositol 3,5-bisphosphate: Metabolism and cellular functions. Trends Biochem. Sci. 2006, 31, 52–63. [Google Scholar] [CrossRef] [PubMed]
- Payrastre, B.; Missy, K.; Giuriato, S.; Bodin, S.; Plantavid, M.; Gratacap, M. Phosphoinositides: Key players in cell signalling, in time and space. Cell Signal. 2001, 13, 377–387. [Google Scholar] [CrossRef]
- Marcus, A.J.; Ullman, H.L.; Safier, L.B. Lipid composition of subcellular particles of human blood platelets. J. Lipid Res. 1969, 10, 108–114. [Google Scholar] [PubMed]
- Trevelyan, W.E. Preparation of phosphatidyl inositol from bakers’ yeast. J. Lipid Res. 1966, 7, 445–447. [Google Scholar] [PubMed]
- Nikawa, J.; Yamashita, S. Molecular cloning of the gene encoding cdpdiacylglycerol-inositol 3-phosphatidyl transferase in saccharomyces cerevisiae. Eur. J. Biochem. 1984, 143, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Antonsson, B.E. Purification and characterization of phosphatidylinositol synthase from human placenta. Biochem. J. 1994, 297, 517–522. [Google Scholar] [CrossRef] [PubMed]
- Laporte, J.; Bedez, F.; Bolino, A.; Mandel, J.L. Myotubularins, a large disease-associated family of cooperating catalytically active and inactive phosphoinositides phosphatases. Hum. Mol. Genet. 2003, 12, R285–R292. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Boukhelifa, M.; Tribble, E.; Bankaitis, V.A. Functional studies of the mammalian Sac1 phosphoinositide phosphatase. Adv. Enzyme Regul. 2009, 49, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Parrish, W.R.; Stefan, C.J.; Emr, S.D. Essential role for the myotubularin-related phosphatase Ymr1p and the synaptojanin-like phosphatases Sjl2p and Sjl3p in regulation of phosphatidylinositol 3-phosphate in yeast. Mol. Biol. Cell 2004, 15, 3567–3579. [Google Scholar] [CrossRef] [PubMed]
- De Matteis, M.; Godi, A.; Corda, D. Phosphoinositides and the Golgi complex. Curr. Opin. Cell Biol. 2002, 14, 434–447. [Google Scholar] [CrossRef]
- Audhya, A.; Foti, M.; Emr, S.D. Distinct roles for the yeast phosphatidylinositol 4-kinases, Stt4p and Pik1p, in secretion, cell growth, and organelle membrane dynamics. Mol. Biol. Cell 2000, 11, 2673–2689. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Bankaitis, V.A. Phosphoinositide phosphatases in cell biology and disease. Prog. Lipid Res. 2010, 49, 201–217. [Google Scholar] [CrossRef] [PubMed]
- Leslie, N.R.; Longy, M. Inherited PTEN mutations and the prediction of phenotype. Semin. Cell Dev. Biol. 2016, 52, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.; Orrison, B.M.; Leahey, A.M.; Suchy, S.F.; Bernard, D.J.; Lewis, R.A.; Nussbaum, R.L. Spectrum of mutations in the OCRL1 gene in the lowe oculocerebrorenal syndrome. Am. J. Hum. Genet. 1997, 60, 1384–1388. [Google Scholar] [CrossRef] [PubMed]
- Bielas, S.L.; Silhavy, J.L.; Brancati, F.; Kisseleva, M.V.; Al-Gazali, L.; Sztriha, L.; Bayoumi, R.A.; Zaki, M.S.; Abdel-Aleem, A.; Rosti, R.O.; et al. Mutations in INPP5E, encoding inositol polyphosphate-5-phosphatase E, link phosphatidyl inositol signaling to the ciliopathies. Nat. Genet. 2009, 41, 1032–1036. [Google Scholar] [CrossRef] [PubMed]
- Jacoby, M.; Cox, J.J.; Gayral, S.; Hampshire, D.J.; Ayub, M.; Blockmans, M.; Pernot, E.; Kisseleva, M.V.; Compere, P.; Schiffmann, S.N.; et al. INPP5E mutations cause primary cilium signaling defects, ciliary instability and ciliopathies in human and mouse. Nat. Genet. 2009, 41, 1027–1031. [Google Scholar] [CrossRef] [PubMed]
- Krebs, C.E.; Karkheiran, S.; Powell, J.C.; Cao, M.; Makarov, V.; Darvish, H.; di Paolo, G.; Walker, R.H.; Shahidi, G.A.; Buxbaum, J.D.; et al. The Sac1 domain of SYNJ1 identified mutated in a family with early-onset progressive parkinsonism with generalized seizures. Hum. Mutat. 2013, 34, 1200–1207. [Google Scholar] [CrossRef] [PubMed]
- Quadri, M.; Fang, M.; Picillo, M.; Olgiati, S.; Breedveld, G.J.; Graafland, J.; Wu, B.; Xu, F.; Erro, R.; Amboni, M.; et al. Mutation in the SYNJ1 gene associated with autosomal recessive, early-onset parkinsonism. Hum. Mutat. 2013, 34, 1208–1215. [Google Scholar] [CrossRef] [PubMed]
- Godi, A.; di Campli, A.; Konstantakopoulos, A.; di Tullio, G.; Alessi, D.R.; Kular, G.S.; Daniele, T.; Marra, P.; Lucocq, J.M.; de Matteis, M.A. FAPPs control Golgi-to-cell-surface membrane traffic by binding to ARF and PtdIns(4)P. Nat. Cell Biol. 2004, 6, 393–404. [Google Scholar] [CrossRef] [PubMed]
- Mizuno-Yamasaki, E.; Medkova, M.; Coleman, J.; Novick, P. Phosphatidylinositol 4-phosphate controls both membrane recruitment and a regulatory switch of the Rab GEF Sec2p. Dev. Cell 2010, 18, 828–840. [Google Scholar] [CrossRef] [PubMed]
- Lemmon, M.A. Phosphoinositide recognition domains. Traffic 2003, 4, 201–213. [Google Scholar] [CrossRef] [PubMed]
- Levine, T.P.; Munro, S. Targeting of Golgi-specific pleckstrin homology domains involves both PtdIns 4-kinase-dependent and -independent components. Curr. Biol. 2002, 12, 695–704. [Google Scholar] [CrossRef]
- De Saint-Jean, M.; Delfosse, V.; Douguet, D.; Chicanne, G.; Payrastre, B.; Bourguet, W.; Antonny, B.; Drin, G. Osh4p exchanges sterols for phosphatidylinositol 4-phosphate between lipid bilayers. J. Cell Biol. 2011, 195, 965–978. [Google Scholar] [CrossRef] [PubMed]
- Moser von Filseck, J.; Copic, A.; Delfosse, V.; Vanni, S.; Jackson, C.L.; Bourguet, W.; Drin, G. Phosphatidylserine transport by ORP/Osh proteins is driven by phosphatidylinositol 4-phosphate. Science 2015, 349, 432–436. [Google Scholar] [CrossRef] [PubMed]
- Mesmin, B.; Bigay, J.; Moser von Filseck, J.; Lacas-Gervais, S.; Drin, G.; Antonny, B. A four-step cycle driven by PI(4)P hydrolysis directs sterol/PI(4)P exchange by the ER-Golgi tether OSBP. Cell 2013, 155, 830–843. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.; Torta, F.; Masai, K.; Lucast, L.; Czapla, H.; Tanner, L.B.; Narayanaswamy, P.; Wenk, M.R.; Nakatsu, F.; de Camilli, P. PI4P/phosphatidylserine countertransport at ORP5- and ORP8-mediated ER-plasma membrane contacts. Science 2015, 349, 428–432. [Google Scholar] [CrossRef] [PubMed]
- Galmes, R.; Houcine, A.; van Vliet, A.R.; Agostinis, P.; Jackson, C.L.; Giordano, F. ORP5/ORP8 localize to endoplasmic reticulum-mitochondria contacts and are involved in mitochondrial function. EMBO Rep. 2016, 17, 800–810. [Google Scholar] [CrossRef] [PubMed]
- Dong, R.; Saheki, Y.; Swarup, S.; Lucast, L.; Harper, J.W.; de Camilli, P. Endosome-ER contacts control actin nucleation and retromer function through VAP-dependent regulation of PI4P. Cell 2016, 166, 408–423. [Google Scholar] [CrossRef] [PubMed]
- Lenoir, M.; Grzybek, M.; Majkowski, M.; Rajesh, S.; Kaur, J.; Whittaker, S.B.; Coskun, U.; Overduin, M. Structural basis of dynamic membrane recognition by trans-Golgi network specific FAPP proteins. J. Mol. Biol. 2015, 427, 966–981. [Google Scholar] [CrossRef] [PubMed]
- Henmi, Y.; Morikawa, Y.; Oe, N.; Ikeda, N.; Fujita, A.; Takei, K.; Minogue, S.; Tanabe, K. PtdIns4KIIα generates endosomal PtdIns(4)P and is required for receptor sorting at early endosomes. Mol. Biol. Cell 2016, 27, 990–1001. [Google Scholar] [CrossRef] [PubMed]
- Chavez, M.; Ena, S.; van Sande, J.; de Kerchove d’Exaerde, A.; Schurmans, S.; Schiffmann, S.N. Modulation of ciliary phosphoinositide content regulates trafficking and sonic hedgehog signaling output. Dev. Cell 2015, 34, 338–350. [Google Scholar] [CrossRef] [PubMed]
- Herman, P.K.; Emr, S.D. Characterization of VPS34, a gene required for vacuolar protein sorting and vacuole segregation in saccharomyces cerevisiae. Mol. Cell Biol. 1990, 10, 6742–6754. [Google Scholar] [CrossRef] [PubMed]
- Schu, P.V.; Takegawa, K.; Fry, M.J.; Stack, J.H.; Waterfield, M.D.; Emr, S.D. Phosphatidylinositol 3-kinase encoded by yeast VPS34 gene essential for protein sorting. Science 1993, 260, 88–91. [Google Scholar] [CrossRef] [PubMed]
- Kihara, A.; Noda, T.; Ishihara, N.; Ohsumi, Y. Two distinct Vps34 phosphatidylinositol 3-kinase complexes function in autophagy and carboxypeptidase Y sorting in saccharomyces cerevisiae. J. Cell Biol. 2001, 152, 519–530. [Google Scholar] [CrossRef] [PubMed]
- Slessareva, J.E.; Routt, S.M.; Temple, B.; Bankaitis, V.A.; Dohlman, H.G. Activation of the phosphatidylinositol 3-kinase Vps34 by a G protein α subunit at the endosome. Cell 2006, 126, 191–203. [Google Scholar] [CrossRef] [PubMed]
- Vanhaesebroeck, B.; Leevers, S.J.; Ahmadi, K.; Timms, J.; Katso, R.; Driscoll, P.C.; Woscholski, R.; Parker, P.J.; Waterfield, M.D. Synthesis and function of 3-phosphorylated inositol lipids. Annu. Rev. Biochem. 2001, 70, 535–602. [Google Scholar] [CrossRef] [PubMed]
- Vanhaesebroeck, B.; Guillermet-Guibert, J.; Graupera, M.; Bilanges, B. The emerging mechanisms of isoform-specific PI3K signalling. Nat. Rev. Mol. Cell Biol. 2010, 11, 329–341. [Google Scholar] [CrossRef] [PubMed]
- Panaretou, C.; Domin, J.; Cockcroft, S.; Waterfield, M.D. Characterization of p150, an adaptor protein for the human phosphatidylinositol (PtdIns) 3-kinase. Substrate presentation by phosphatidylinositol transfer protein to the p150.Ptdins 3-kinase complex. J. Biol. Chem. 1997, 272, 2477–2485. [Google Scholar] [PubMed]
- Lecompte, O.; Poch, O.; Laporte, J. PtdIns5P regulation through evolution: Roles in membrane trafficking? Trends Biochem. Sci. 2008, 33, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Stoetzel, C.; Bar, S.; de Craene, J.O.; Scheidecker, S.; Etard, C.; Chicher, J.; Reck, J.R.; Perrault, I.; Geoffroy, V.; Chennen, K.; et al. A mutation in VPS15 (PIK3R4) causes a ciliopathy and affects IFT20 release from the cis-Golgi. Nat. Commun. 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Gary, J.D.; Sato, T.K.; Stefan, C.J.; Bonangelino, C.J.; Weisman, L.S.; Emr, S.D. Regulation of Fab1 phosphatidylinositol 3-phosphate 5-kinase pathway by Vac7 protein and Fig4, a polyphosphoinositide phosphatase family member. Mol. Biol. Cell 2002, 13, 1238–1251. [Google Scholar] [CrossRef] [PubMed]
- Hnia, K.; Vaccari, I.; Bolino, A.; Laporte, J. Myotubularin phosphoinositide phosphatases: Cellular functions and disease pathophysiology. Trends Mol. Med. 2012, 18, 317–327. [Google Scholar] [CrossRef] [PubMed]
- Nicot, A.S.; Laporte, J. Endosomal phosphoinositides and human diseases. Traffic 2008, 9, 1240–1249. [Google Scholar] [CrossRef] [PubMed]
- Sabha, N.; Volpatti, J.R.; Gonorazky, H.; Reifler, A.; Davidson, A.E.; Li, X.; Eltayeb, N.M.; Dall’Armi, C.; di Paolo, G.; Brooks, S.V.; et al. PIK3C2B inhibition improves function and prolongs survival in myotubular myopathy animal models. J. Clin. Investig. 2016, 126, 3613–3625. [Google Scholar] [CrossRef] [PubMed]
- Henne, W.M.; Buchkovich, N.J.; Emr, S.D. The ESCRT pathway. Dev. Cell 2011, 21, 77–91. [Google Scholar] [CrossRef] [PubMed]
- Obara, K.; Ohsumi, Y. PtdIns 3-kinase orchestrates autophagosome formation in yeast. J. Lipids 2011, 2011. [Google Scholar] [CrossRef] [PubMed]
- Baskaran, S.; Ragusa, M.J.; Boura, E.; Hurley, J.H. Two-site recognition of phosphatidylinositol 3-phosphate by proppins in autophagy. Mol. Cell 2012, 47, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Gillooly, D.J.; Morrow, I.C.; Lindsay, M.; Gould, R.; Bryant, N.J.; Gaullier, J.M.; Parton, R.G.; Stenmark, H. Localization of phosphatidylinositol 3-phosphate in yeast and mammalian cells. EMBO J. 2000, 19, 4577–4588. [Google Scholar] [CrossRef] [PubMed]
- Gruenberg, J.; Stenmark, H. The biogenesis of multivesicular endosomes. Nat. Rev. Mol. Cell Biol. 2004, 5, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Hortsman, H.; Seet, L.; Wong, S.H.; Hong, W. SNX3 regulates endosomal function through its PX-domain-mediated interaction with PtdIns(3)P. Nat. Cell Biol. 2001, 3, 658–666. [Google Scholar] [CrossRef] [PubMed]
- Pons, V.; Luyet, P.P.; Morel, E.; Abrami, L.; van der Goot, F.G.; Parton, R.G.; Gruenberg, J. Hrs and SNX3 functions in sorting and membrane invagination within multivesicular bodies. PLoS Biol. 2008, 6, e214. [Google Scholar] [CrossRef] [PubMed]
- Teasdale, R.D.; Collins, B.M. Insights into the PX (phox-homology) domain and SNX (sorting nexin) protein families: Structures, functions and roles in disease. Biochem. J. 2012, 441, 39–59. [Google Scholar] [CrossRef] [PubMed]
- Van Weering, J.R.; Verkade, P.; Cullen, P.J. SNX-BAR proteins in phosphoinositide-mediated, tubular-based endosomal sorting. Semin. Cell Dev. Biol. 2010, 21, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Hamasaki, M.; Furuta, N.; Matsuda, A.; Nezu, A.; Yamamoto, A.; Fujita, N.; Oomori, H.; Noda, T.; Haraguchi, T.; Hiraoka, Y.; et al. Autophagosomes form at ER-mitochondria contact sites. Nature 2013, 495, 389–393. [Google Scholar] [CrossRef] [PubMed]
- Axe, E.L.; Walker, S.A.; Manifava, M.; Chandra, P.; Roderick, H.L.; Habermann, A.; Griffiths, G.; Ktistakis, N.T. Autophagosome formation from membrane compartments enriched in phosphatidylinositol 3-phosphate and dynamically connected to the endoplasmic reticulum. J. Cell Biol. 2008, 182, 685–701. [Google Scholar] [CrossRef] [PubMed]
- Polson, H.E.; de Lartigue, J.; Rigden, D.J.; Reedijk, M.; Urbe, S.; Clague, M.J.; Tooze, S.A. Mammalian Atg18 (WIPI2) localizes to omegasome-anchored phagophores and positively regulates LC3 lipidation. Autophagy 2010, 6, 506–522. [Google Scholar] [CrossRef] [PubMed]
- Proikas-Cezanne, T.; Takacs, Z.; Donnes, P.; Kohlbacher, O. Wipi proteins: Essential PtdIns3P effectors at the nascent autophagosome. J. Cell Sci. 2015, 128, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Dooley, H.C.; Razi, M.; Polson, H.E.; Girardin, S.E.; Wilson, M.I.; Tooze, S.A. WIPI2 links LC3 conjugation with PI3P, autophagosome formation, and pathogen clearance by recruiting Atg12-5-16L1. Mol. Cell 2014, 55, 238–252. [Google Scholar] [CrossRef] [PubMed]
- Mohl, B.P.; Bartlett, C.; Mankouri, J.; Harris, M. Early events in the generation of autophagosomes are required for the formation of membrane structures involved in hepatitis C virus genome replication. J. Gen. Virol. 2016, 97, 680–693. [Google Scholar] [CrossRef] [PubMed]
- Rameh, L.E.; Tolias, K.F.; Duckworth, B.C.; Cantley, L.C. A new pathway for synthesis of phosphatidylinositol-4,5-bisphosphate. Nature 1997, 390, 192–196. [Google Scholar] [CrossRef] [PubMed]
- Sarkes, D.; Rameh, L.E. A novel HPLC-based approach makes possible the spatial characterization of cellular PtdIns5P and other phosphoinositides. Biochem. J. 2010, 428, 375–384. [Google Scholar] [CrossRef] [PubMed]
- Sbrissa, D.; Ikonomov, O.C.; Deeb, R.; Shisheva, A. Phosphatidylinositol 5-phosphate biosynthesis is linked to PIKfyve and is involved in osmotic response pathway in mammalian cells. J. Biol. Chem. 2002, 277, 47276–47284. [Google Scholar] [CrossRef] [PubMed]
- Coronas, S.; Lagarrigue, F.; Ramel, D.; Chicanne, G.; Delsol, G.; Payrastre, B.; Tronchere, H. Elevated levels of PtdIns5P in NPM-ALK transformed cells: Implication of PIKfyve. Biochem. Biophys. Res. Commun. 2008, 372, 351–355. [Google Scholar] [CrossRef] [PubMed]
- Ikonomov, O.C.; Sbrissa, D.; Delvecchio, K.; Xie, Y.; Jin, J.P.; Rappolee, D.; Shisheva, A. The phosphoinositide kinase PIKfyve is vital in early embryonic development: Preimplantation lethality of PIKfyve−/− embryos but normality of PIKfyve+/− mice. J. Biol. Chem. 2011, 286, 13404–13413. [Google Scholar] [CrossRef] [PubMed]
- Oppelt, A.; Lobert, V.H.; Haglund, K.; Mackey, A.M.; Rameh, L.E.; Liestol, K.; Schink, K.O.; Pedersen, N.M.; Wenzel, E.M.; Haugsten, E.M.; et al. Production of phosphatidylinositol 5-phosphate via PIKfyve and MTMR3 regulates cell migration. EMBO Rep. 2013, 14, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Raess, M.A.; Friant, S.; Cowling, B.S.; Laporte, J. Wanted - dead or alive: Myotubularins, a large disease-associated protein family. Adv. Biol. Regul. 2017, 63, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Tronchere, H.; Laporte, J.; Pendaries, C.; Chaussade, C.; Liaubet, L.; Pirola, L.; Mandel, J.L.; Payrastre, B. Production of phosphatidylinositol 5-phosphate by the phosphoinositide 3-phosphatase myotubularin in mammalian cells. J. Biol. Chem. 2004, 279, 7304–7312. [Google Scholar] [CrossRef] [PubMed]
- Niebuhr, K.; Giuriato, S.; Pedron, T.; Philpott, D.J.; Gaits, F.; Sable, J.; Sheetz, M.P.; Parsot, C.; Sansonetti, P.J.; Payrastre, B. Conversion of PtdIns(4,5)P(2) into PtdIns(5)P by the S.flexneri effector IpgD reorganizes host cell morphology. EMBO J. 2002, 21, 5069–5078. [Google Scholar] [CrossRef] [PubMed]
- Taylor, G.S.; Maehama, T.; Dixon, J.E. Inaugural article: Myotubularin, a protein tyrosine phosphatase mutated in myotubular myopathy, dephosphorylates the lipid second messenger, phosphatidylinositol 3-phosphate. Proc. Natl. Acad. Sci. USA 2000, 97, 8910–8915. [Google Scholar] [CrossRef] [PubMed]
- Gozani, O.; Karuman, P.; Jones, D.R.; Ivanov, D.; Cha, J.; Lugovskoy, A.A.; Baird, C.L.; Zhu, H.; Field, S.J.; Lessnick, S.L.; et al. The PHD finger of the chromatin-associated protein ING2 functions as a nuclear phosphoinositide receptor. Cell 2003, 114, 99–111. [Google Scholar] [CrossRef]
- Guittard, G.; Gerard, A.; Dupuis-Coronas, S.; Tronchere, H.; Mortier, E.; Favre, C.; Olive, D.; Zimmermann, P.; Payrastre, B.; Nunes, J.A. Cutting edge: Dok-1 and Dok-2 adaptor molecules are regulated by phosphatidylinositol 5-phosphate production in T cells. J. Immunol. 2009, 182, 3974–3978. [Google Scholar] [CrossRef] [PubMed]
- Viaud, J.; Lagarrigue, F.; Ramel, D.; Allart, S.; Chicanne, G.; Ceccato, L.; Courilleau, D.; Xuereb, J.M.; Pertz, O.; Payrastre, B.; et al. Phosphatidylinositol 5-phosphate regulates invasion through binding and activation of Tiam1. Nat. Commun. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Boal, F.; Mansour, R.; Gayral, M.; Saland, E.; Chicanne, G.; Xuereb, J.M.; Marcellin, M.; Burlet-Schiltz, O.; Sansonetti, P.J.; Payrastre, B.; et al. TOM1 is a PI5P effector involved in the regulation of endosomal maturation. J. Cell Sci. 2015, 128, 815–827. [Google Scholar] [CrossRef] [PubMed]
- Vicinanza, M.; Korolchuk, V.I.; Ashkenazi, A.; Puri, C.; Menzies, F.M.; Clarke, J.H.; Rubinsztein, D.C. PI(5)P regulates autophagosome biogenesis. Mol. Cell 2015, 57, 219–234. [Google Scholar] [CrossRef] [PubMed]
- Di Paolo, G.; de Camilli, P. Phosphoinositides in cell regulation and membrane dynamics. Nature 2006, 443, 651–657. [Google Scholar] [CrossRef] [PubMed]
- Desrivieres, S.; Cooke, F.T.; Parker, P.J.; Hall, M.N. MSS4, a phosphatidylinositol-4-phosphate 5-kinase required for organization of the actin cytoskeleton in Saccharomyces cerevisiae. J. Biol. Chem. 1998, 273, 15787–15793. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, H.; Shibasaki, Y.; Kizuki, N.; Wada, T.; Yazaki, Y.; Asano, T.; Oka, Y. Type i phosphatidylinositol-4-phosphate 5-kinases. Cloning of the third isoform and deletion/substitution analysis of members of this novel lipid kinase family. J. Biol. Chem. 1998, 273, 8741–8748. [Google Scholar] [CrossRef] [PubMed]
- Bunce, M.W.; Boronenkov, I.V.; Anderson, R.A. Coordinated activation of the nuclear ubiquitin ligase Cul3-SPOP by the generation of phosphatidylinositol 5-phosphate. J. Biol. Chem. 2008, 283, 8678–8686. [Google Scholar] [CrossRef] [PubMed]
- Clarke, J.H.; Emson, P.C.; Irvine, R.F. Localization of phosphatidylinositol phosphate kinase IIγ in kidney to a membrane trafficking compartment within specialized cells of the nephron. Am. J. Physiol. Renal. Physiol. 2008, 295, F1422–F1430. [Google Scholar] [CrossRef] [PubMed]
- Lacalle, R.A.; de Karam, J.C.; Martinez-Munoz, L.; Artetxe, I.; Peregil, R.M.; Sot, J.; Rojas, A.M.; Goni, F.M.; Mellado, M.; Manes, S. Type I phosphatidylinositol 4-phosphate 5-kinase homo- and heterodimerization determines its membrane localization and activity. FASEB J. 2015, 29, 2371–2385. [Google Scholar] [CrossRef] [PubMed]
- Audhya, A.; Emr, S.D. Stt4 PI 4-kinase localizes to the plasma membrane and functions in the Pkc1-mediated MAP kinase cascade. Dev. Cell 2002, 2, 593–605. [Google Scholar] [CrossRef]
- Sun, Y.; Carroll, S.; Kaksonen, M.; Toshima, J.Y.; Drubin, D.G. PtdIns(4,5)P2 turnover is required for multiple stages during clathrin- and actin-dependent endocytic internalization. J. Cell Biol. 2007, 177, 355–367. [Google Scholar] [CrossRef] [PubMed]
- Itoh, T.; Takenawa, T. Phosphoinositide-binding domains: Functional units for temporal and spatial regulation of intracellular signalling. Cell Signal. 2002, 14, 733–743. [Google Scholar] [CrossRef]
- De Craene, J.O.; Ripp, R.; Lecompte, O.; Thompson, J.D.; Poch, O.; Friant, S. Evolutionary analysis of the ENTH/ANTH/VHS protein superfamily reveals a coevolution between membrane trafficking and metabolism. BMC Genom. 2012, 13. [Google Scholar] [CrossRef] [PubMed]
- Cortesio, C.L.; Lewellyn, E.B.; Drubin, D.G. Control of lipid organization and actin assembly during clathrin-mediated endocytosis by the cytoplasmic tail of the rhomboid protein Rbd2. Mol. Biol. Cell 2015, 26, 1509–1522. [Google Scholar] [CrossRef] [PubMed]
- Soulard, A.; Friant, S.; Fitterer, C.; Orange, C.; Kaneva, G.; Mirey, G.; Winsor, B. The WASP/Las17p-interacting protein Bzz1p functions with Myo5p in an early stage of endocytosis. Protoplasma 2005, 226, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Ford, M.G.; Pearse, B.M.; Higgins, M.K.; Vallis, Y.; Owen, D.J.; Gibson, A.; Hopkins, C.R.; Evans, P.R.; McMahon, H.T. Simultaneous binding of PtdIns(4,5)P2 and clathrin by AP180 in the nucleation of clathrin lattices on membranes. Science 2001, 291, 1051–1055. [Google Scholar] [CrossRef] [PubMed]
- Koharudin, L.M.; Furey, W.; Liu, H.; Liu, Y.J.; Gronenborn, A.M. The phox domain of sorting nexin 5 lacks phosphatidylinositol 3-phosphate (PtdIns(3)P) specificity and preferentially binds to phosphatidylinositol 4,5-bisphosphate (PtdIns(4,5)P2). J. Biol. Chem. 2009, 284, 23697–23707. [Google Scholar] [CrossRef] [PubMed]
- Gallop, J.L.; Walrant, A.; Cantley, L.C.; Kirschner, M.W. Phosphoinositides and membrane curvature switch the mode of actin polymerization via selective recruitment of toca-1 and Snx9. Proc. Natl. Acad. Sci. USA 2013, 110, 7193–7198. [Google Scholar] [CrossRef] [PubMed]
- Nandez, R.; Balkin, D.M.; Messa, M.; Liang, L.; Paradise, S.; Czapla, H.; Hein, M.Y.; Duncan, J.S.; Mann, M.; de Camilli, P. A role of OCRL in clathrin-coated pit dynamics and uncoating revealed by studies of lowe syndrome cells. Elife 2014, 3. [Google Scholar] [CrossRef] [PubMed]
- Cauvin, C.; Rosendale, M.; Gupta-Rossi, N.; Rocancourt, M.; Larraufie, P.; Salomon, R.; Perrais, D.; Echard, A. Rab35 GTPase triggers switch-like recruitment of the lowe syndrome lipid phosphatase OCRL on newborn endosomes. Curr. Biol. 2016, 26, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Merino-Trigo, A.; Kerr, M.C.; Houghton, F.; Lindberg, A.; Mitchell, C.; Teasdale, R.D.; Gleeson, P.A. Sorting nexin 5 is localized to a subdomain of the early endosomes and is recruited to the plasma membrane following EGF stimulation. J. Cell Sci. 2004, 117, 6413–6424. [Google Scholar] [CrossRef] [PubMed]
- Hilpela, P.; Vartiainen, M.K.; Lappalainen, P. Regulation of the actin cytoskeleton by PI(4,5)P2 and PI(3,4,5)P3. Curr. Top. Microbiol. Immunol. 2004, 282, 117–163. [Google Scholar] [PubMed]
- Mattila, P.K.; Pykalainen, A.; Saarikangas, J.; Paavilainen, V.O.; Vihinen, H.; Jokitalo, E.; Lappalainen, P. Missing-in-metastasis and IRSp53 deform PI(4,5)P2-rich membranes by an inverse BAR domain-like mechanism. J. Cell. Biol. 2007, 176, 953–964. [Google Scholar] [CrossRef] [PubMed]
- Santarius, M.; Lee, C.H.; Anderson, R.A. Supervised membrane swimming: Small G-protein lifeguards regulate PIPK signalling and monitor intracellular PtdIns(4,5)P2 pools. Biochem. J. 2006, 398, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Osborne, S.L.; Thomas, C.L.; Gschmeissner, S.; Schiavo, G. Nuclear PtdIns(4,5)P2 assembles in a mitotically regulated particle involved in pre-mRNA splicing. J. Cell Sci. 2001, 114, 2501–2511. [Google Scholar] [PubMed]
- Li, W.; Laishram, R.S.; Anderson, R.A. The novel poly(A) polymerase star-PAP is a signal-regulated switch at the 3′-end of mRNAs. Adv. Biol. Regul. 2013, 53, 64–76. [Google Scholar] [CrossRef] [PubMed]
- Sengelaub, C.A.; Navrazhina, K.; Ross, J.B.; Halberg, N.; Tavazoie, S.F. PTPRN2 and PLCβ1 promote metastatic breast cancer cell migration through PI(4,5)P2-dependent actin remodeling. EMBO J. 2016, 35, 62–76. [Google Scholar] [CrossRef] [PubMed]
- Takatori, S.; Tatematsu, T.; Cheng, J.; Matsumoto, J.; Akano, T.; Fujimoto, T. Phosphatidylinositol 3,5-bisphosphate-rich membrane domains in endosomes and lysosomes. Traffic 2016, 17, 154–167. [Google Scholar] [CrossRef] [PubMed]
- Dove, S.K.; Cooke, F.T.; Douglas, M.R.; Sayers, L.G.; Parker, P.J.; Michell, R.H. Osmotic stress activates phosphatidylinositol-3,5-bisphosphate synthesis. Nature 1997, 390, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Bonangelino, C.J.; Nau, J.J.; Duex, J.E.; Brinkman, M.; Wurmser, A.E.; Gary, J.D.; Emr, S.D.; Weisman, L.S. Osmotic stress-induced increase of phosphatidylinositol 3,5-bisphosphate requires Vac14p, an activator of the lipid kinase Fab1p. J. Cell Biol. 2002, 156, 1015–1028. [Google Scholar] [CrossRef] [PubMed]
- Duex, J.E.; Tang, F.; Weisman, L.S. The Vac14p-Fig4p complex acts independently of Vac7p and couples PI3,5P2 synthesis and turnover. J. Cell Biol. 2006, 172, 693–704. [Google Scholar] [CrossRef] [PubMed]
- Duex, J.E.; Nau, J.J.; Kauffman, E.J.; Weisman, L.S. Phosphoinositide 5-phosphatase Fig 4p is required for both acute rise and subsequent fall in stress-induced phosphatidylinositol 3,5-bisphosphate levels. Eukaryot. Cell 2006, 5, 723–731. [Google Scholar] [CrossRef] [PubMed]
- Shisheva, A. Pikfyve: Partners, significance, debates and paradoxes. Cell. Biol. Int. 2008, 32, 591–604. [Google Scholar] [CrossRef] [PubMed]
- Ikonomov, O.C.; Sbrissa, D.; Fligger, J.; Delvecchio, K.; Shisheva, A. ArPIKfyve regulates Sac3 protein abundance and turnover: Disruption of the mechanism by Sac3I41T mutation causing Charcot-Marie-Tooth 4J disorder. J. Biol. Chem. 2010, 285, 26760–26764. [Google Scholar] [CrossRef] [PubMed]
- Jin, N.; Chow, C.Y.; Liu, L.; Zolov, S.N.; Bronson, R.; Davisson, M.; Petersen, J.L.; Zhang, Y.; Park, S.; Duex, J.E.; et al. VAC14 nucleates a protein complex essential for the acute interconversion of PI3P and PI(3,5)P(2) in yeast and mouse. EMBO J. 2008, 27, 3221–3234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chow, C.Y.; Zhang, Y.; Dowling, J.J.; Jin, N.; Adamska, M.; Shiga, K.; Szigeti, K.; Shy, M.E.; Li, J.; Zhang, X.; et al. Mutation of FIG4 causes neurodegeneration in the pale tremor mouse and patients with CMT4J. Nature 2007, 448, 68–72. [Google Scholar] [CrossRef] [PubMed]
- Vaccari, I.; Dina, G.; Tronchere, H.; Kaufman, E.; Chicanne, G.; Cerri, F.; Wrabetz, L.; Payrastre, B.; Quattrini, A.; Weisman, L.S.; et al. Genetic interaction between MTMR2 and FIG4 phospholipid phosphatases involved in Charcot-Marie-Tooth neuropathies. PLoS Genet. 2011, 7, e1002319. [Google Scholar] [CrossRef] [PubMed]
- Chow, C.Y.; Landers, J.E.; Bergren, S.K.; Sapp, P.C.; Grant, A.E.; Jones, J.M.; Everett, L.; Lenk, G.M.; McKenna-Yasek, D.M.; Weisman, L.S.; et al. Deleterious variants of FIG4, a phosphoinositide phosphatase, in patients with ALS. Am. J. Hum. Genet. 2009, 84, 85–88. [Google Scholar] [CrossRef] [PubMed]
- Lenk, G.M.; Szymanska, K.; Debska-Vielhaber, G.; Rydzanicz, M.; Walczak, A.; Bekiesinska-Figatowska, M.; Vielhaber, S.; Hallmann, K.; Stawinski, P.; Buehring, S.; et al. Biallelic mutations of VAC14 in pediatric-onset neurological disease. Am. J. Hum. Genet. 2016, 99, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, A.; DeWald, D.B.; Boronenkov, I.V.; Anderson, R.A.; Emr, S.D.; Koshland, D. Novel PI(4)P 5-kinase homologue, Fab1p, essential for normal vacuole function and morphology in yeast. Mol. Biol. Cell 1995, 6, 525–539. [Google Scholar] [CrossRef] [PubMed]
- Odorizzi, G.; Babst, M.; Emr, S.D. Fab1p PtdIns(3)P 5-kinase function essential for protein sorting in the multivesicular body. Cell 1998, 95, 847–858. [Google Scholar] [CrossRef]
- Whitley, P.; Reaves, B.J.; Hashimoto, M.; Riley, A.M.; Potter, B.V.; Holman, G.D. Identification of mammalian Vps24p as an effector of phosphatidylinositol 3,5-bisphosphate-dependent endosome compartmentalization. J. Biol. Chem. 2003, 278, 38786–38795. [Google Scholar] [CrossRef] [PubMed]
- Friant, S.; Pecheur, E.I.; Eugster, A.; Michel, F.; Lefkir, Y.; Nourrisson, D.; Letourneur, F. Ent3p is a Ptdins(3,5)P2 effector required for protein sorting to the multivesicular body. Dev. Cell 2003, 5, 499–511. [Google Scholar] [CrossRef]
- Eugster, A.; Pecheur, E.I.; Michel, F.; Winsor, B.; Letourneur, F.; Friant, S. Ent5p is required with Ent3p and Vps27p for ubiquitin-dependent protein sorting into the multivesicular body. Mol. Biol. Cell 2004, 15, 3031–3041. [Google Scholar] [CrossRef] [PubMed]
- Chidambaram, S.; Zimmermann, J.; von Mollard, G.F. ENTH domain proteins are cargo adaptors for multiple SNARE proteins at the TGN endosome. J Cell Sci 2008, 121, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Dove, S.K.; Piper, R.C.; McEwen, R.K.; Yu, J.W.; King, M.C.; Hughes, D.C.; Thuring, J.; Holmes, A.B.; Cooke, F.T.; Michell, R.H.; et al. Svp1p defines a family of phosphatidylinositol 3,5-bisphosphate effectors. EMBO J. 2004, 23, 1922–1933. [Google Scholar] [CrossRef] [PubMed]
- Efe, J.A.; Botelho, R.J.; Emr, S.D. Atg18 regulates organelle morphology and Fab1 kinase activity independent of its membrane recruitment by phosphatidylinositol 3,5-bisphosphate. Mol. Biol. Cell 2007, 18, 4232–4244. [Google Scholar] [CrossRef] [PubMed]
- Hong, N.H.; Qi, A.; Weaver, A.M. PI(3,5)P2 controls endosomal branched actin dynamics by regulating cortactin-actin interactions. J. Cell Biol. 2015, 210, 753–769. [Google Scholar] [CrossRef] [PubMed]
- Mironova, Y.A.; Lenk, G.M.; Lin, J.P.; Lee, S.J.; Twiss, J.L.; Vaccari, I.; Bolino, A.; Havton, L.A.; Min, S.H.; Abrams, C.S.; et al. PI(3,5)P2 biosynthesis regulates oligodendrocyte differentiation by intrinsic and extrinsic mechanisms. Elife 2016, 5. [Google Scholar] [CrossRef] [PubMed]
- Misawa, H.; Ohtsubo, M.; Copeland, N.G.; Gilbert, D.J.; Jenkins, N.A.; Yoshimura, A. Cloning and characterization of a novel class II phosphoinositide 3-kinase containing C2 domain. Biochem. Biophys. Res. Commun. 1998, 244, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Edlich, C.; Stier, G.; Simon, B.; Sattler, M.; Muhle-Goll, C. Structure and phosphatidylinositol-(3,4)-bisphosphate binding of the C-terminal PH domain of human pleckstrin. Structure 2005, 13, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Carpten, J.D.; Faber, A.L.; Horn, C.; Donoho, G.P.; Briggs, S.L.; Robbins, C.M.; Hostetter, G.; Boguslawski, S.; Moses, T.Y.; Savage, S.; et al. A transforming mutation in the pleckstrin homology domain of AKT1 in cancer. Nature 2007, 448, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Hers, I.; Vincent, E.E.; Tavare, J.M. Akt signalling in health and disease. Cell Signal. 2011, 23, 1515–1527. [Google Scholar] [CrossRef] [PubMed]
- Woolley, J.F.; Dzneladze, I.; Salmena, L. Phosphoinositide signaling in cancer: INPP4B Akt(s) out. Trends Mol. Med. 2015, 21, 530–532. [Google Scholar] [CrossRef] [PubMed]
- Dowler, S.; Currie, R.A.; Campbell, D.G.; Deak, M.; Kular, G.; Downes, C.P.; Alessi, D.R. Identification of pleckstrin-homology-domain-containing proteins with novel phosphoinositide-binding specificities. Biochem. J. 2000, 351, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.C.; Dowler, S.; Deak, M.; Alessi, D.R.; van Aalten, D.M. Crystal structure of the phosphatidylinositol 3,4-bisphosphate-binding pleckstrin homology (PH) domain of tandem PH-domain-containing protein 1 (TAPP1): Molecular basis of lipid specificity. Biochem. J. 2001, 358, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Wullschleger, S.; Wasserman, D.H.; Gray, A.; Sakamoto, K.; Alessi, D.R. Role of TAPP1 and TAPP2 adaptor binding to PtdIns(3,4)P2 in regulating insulin sensitivity defined by knock-in analysis. Biochem. J. 2011, 434, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Milne, S.B.; Ivanova, P.T.; DeCamp, D.; Hsueh, R.C.; Brown, H.A. A targeted mass spectrometric analysis of phosphatidylinositol phosphate species. J. Lipid Res. 2005, 46, 1796–1802. [Google Scholar] [CrossRef] [PubMed]
- Zheng, P.; Baibakov, B.; Wang, X.H.; Dean, J. PtdIns(3,4,5)P3 is constitutively synthesized and required for spindle translocation during meiosis in mouse oocytes. J. Cell Sci. 2013, 126, 715–721. [Google Scholar] [CrossRef] [PubMed]


© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Craene, J.-O.; Bertazzi, D.L.; Bär, S.; Friant, S. Phosphoinositides, Major Actors in Membrane Trafficking and Lipid Signaling Pathways. Int. J. Mol. Sci. 2017, 18, 634. https://doi.org/10.3390/ijms18030634
De Craene J-O, Bertazzi DL, Bär S, Friant S. Phosphoinositides, Major Actors in Membrane Trafficking and Lipid Signaling Pathways. International Journal of Molecular Sciences. 2017; 18(3):634. https://doi.org/10.3390/ijms18030634
Chicago/Turabian StyleDe Craene, Johan-Owen, Dimitri L. Bertazzi, Séverine Bär, and Sylvie Friant. 2017. "Phosphoinositides, Major Actors in Membrane Trafficking and Lipid Signaling Pathways" International Journal of Molecular Sciences 18, no. 3: 634. https://doi.org/10.3390/ijms18030634