The Cluster [Re6Se8I6]3− Induces Low Hemolysis of Human Erythrocytes in Vitro: Protective Effect of Albumin
Abstract
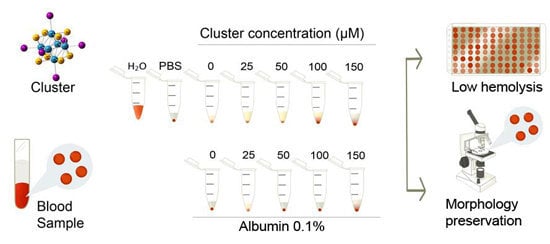
:1. Introduction
2. Results and Discussion




3. Experimental Section
3.1. Synthesis of ([n-Bu4N]3[Re6Se8I6])3−
3.2. Dose Selection
3.3. Human Blood Samples and Preparation of Erythrocytes
3.4. Test of Hemolysis

3.5. Morphology of Erythrocytes
3.6. Data Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Echeverría, C.; Becerra, A.; Nuñez-Villena, F.; Muñoz-Castro, A.; Stehberg, J.; Zheng, Z.; Arratia-Perez, R.; Simon, F.; Ramírez-Tagle, R. The paramagnetic and luminescent [Re6Se8I6]3− cluster. Its potential use as an antitumoral and biomarker agent. New J. Chem. 2012, 36, 927–932. [Google Scholar] [CrossRef]
- Choi, S.-J.; Brylev, K. a; Xu, J.-Z.; Mironov, Y. V.; Fedorov, V.E.; Fedorov, V.E.; Sohn, Y.S.; Kim, S.-J.; Choy, J.-H. Cellular uptake and cytotoxicity of octahedral rhenium cluster complexes. J. Inorg. Biochem. 2008, 102, 1991–1996. [Google Scholar] [CrossRef] [PubMed]
- Shestopalov, M.A.; Zubareva, K.E.; Khripko, O.P.; Khripko, Y.I.; Solovieva, A.O.; Kuratieva, N.V; Mironov, Y.V; Kitamura, N.; Fedorov, V.E.; Brylev, K.A. The first water-soluble hexarhenium cluster complexes with a heterocyclic ligand environment: Synthesis, luminescence, and biological properties. Inorg. Chem. 2014, 53, 9006–9013. [Google Scholar] [CrossRef] [PubMed]
- Aubert, T.; Ledneva, A.Y.; Grasset, F.; Kimoto, K.; Naumov, N.G.; Molard, Y.; Saito, N.; Haneda, H.; Cordier, S. Synthesis and characterization of A4[Re6Q8L6]@SiO2 red-emitting silica nanoparticles based on Re6 metal atom clusters (A = Cs or K, Q = S or Se, and L = OH or CN). Langmuir 2010, 26, 18512–18518. [Google Scholar] [CrossRef] [PubMed]
- Brylev, K.; Shestopalov, M. Biodistribution of Rhenium Cluster Complex K4 in the Body of Laboratory Rats. Bull. Exp. 2013, 155, 741–744. [Google Scholar] [CrossRef]
- Jin, X.; Chalmers, J.J.; Zborowski, M. Iron transport in cancer cell culture suspensions measured by cell magnetophoresis. Anal. Chem. 2012, 84, 4520–4526. [Google Scholar] [CrossRef] [PubMed]
- Love, S.A.; Thompson, J.W.; Haynes, C.L. Development of screening assays for nanoparticle toxicity assessment in human blood: Preliminary studies with charged Au nanoparticles. Nanomedicine (Lond.) 2012, 7, 1355–1364. [Google Scholar] [CrossRef]
- Asharani, P.V.; Sethu, S.; Vadukumpully, S.; Zhong, S.; Lim, C.T.; Hande, M.P.; Valiyaveettil, S. Investigations on the structural damage in human erythrocytes exposed to silver, gold, and platinum nanoparticles. Adv. Funct. Mater. 2010, 20, 1233–1242. [Google Scholar] [CrossRef]
- Liao, K.-H.; Lin, Y.-S.; Macosko, C.W.; Haynes, C.L. Cytotoxicity of graphene oxide and graphene in human erythrocytes and skin fibroblasts. ACS Appl. Mater. Interfaces 2011, 3, 2607–2615. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Xiong, W.; Zhu, Y.; Xu, H.; Yang, X. Protective effect of PEGylation against poly (amidoamine) dendrimer-induced hemolysis of human red blood cells. J. Biomed. Mater. Res. B. Appl. Biomater. 2010, 93, 59–64. [Google Scholar] [PubMed]
- Kandagal, P.B.; Ashoka, S.; Seetharamappa, J.; Shaikh, S.M.T.; Jadegoud, Y.; Ijare, O.B. Study of the interaction of an anticancer drug with human and bovine serum albumin: Spectroscopic approach. J. Pharm. Biomed. Anal. 2006, 41, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Sułkowska, A. Interaction of drugs with bovine and human serum albumin. J. Mol. Struct. 2002, 614, 227–232. [Google Scholar] [CrossRef]
- Espósito, B.P.; Najjar, R. Interactions of antitumoral platinum-group metallodrugs with albumin. Coord. Chem. Rev. 2002, 232, 137–149. [Google Scholar] [CrossRef]
- Chrysanthakopoulos, M.; Giaginis, C.; Tsantili-Kakoulidou, A. Retention of structurally diverse drugs in human serum albumin chromatography and its potential to simulate plasma protein binding. J. Chromatogr. A 2010, 1217, 5761–5768. [Google Scholar] [CrossRef] [PubMed]
- Dobrovolskaia, M.A; Clogston, J.D.; Neun, B.W.; Hall, J.B.; Patri, A.K.; McNeil, S.E. Method for analysis of nanoparticle hemolytic properties in vitro. Nano Lett. 2008, 8, 2180–2187. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, M.; Chakraborty, A.; Mukherjee, A. Cytotoxic, genotoxic and the hemolytic effect of titanium dioxide (TiO2) nanoparticles on human erythrocyte and lymphocyte cells in vitro. J. Appl. Toxicol. 2013, 33, 1097–1110. [Google Scholar] [CrossRef] [PubMed]
- Hamoudeh, M.; Fessi, H.; Mehier, H.; Al Faraj, A.; Canet-Soulas, E. Dirhenium decacarbonyl-loaded PLLA nanoparticles: influence of neutron irradiation and preliminary in vivo administration by the TMT technique. Int. J. Pharm. 2008, 348, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Orto, P.J.; Nichol, G.S.; Okumura, N.; Evans, D.H.; Arratia-Pérez, R.; Ramirez-Tagle, R.; Wang, R.; Zheng, Z. Cluster carbonyls of the [Re6Se8I6]3− core: Synthesis, structural characterization, and computational analysis. Dalton Trans. 2008, 6, 4247–4253. [Google Scholar] [CrossRef]
- Zheng, Z.; Gray, T.G.; Holm, R.H. Synthesis and structures of solvated monoclusters and bridged Di- and triclusters based on the cubic building block [Re6Se8I6]3−. Inorg. Chem. 1999, 38, 4888–4895. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rojas-Mancilla, E.; Oyarce, A.; Verdugo, V.; Zheng, Z.; Ramírez-Tagle, R. The Cluster [Re6Se8I6]3− Induces Low Hemolysis of Human Erythrocytes in Vitro: Protective Effect of Albumin. Int. J. Mol. Sci. 2015, 16, 1728-1735. https://doi.org/10.3390/ijms16011728
Rojas-Mancilla E, Oyarce A, Verdugo V, Zheng Z, Ramírez-Tagle R. The Cluster [Re6Se8I6]3− Induces Low Hemolysis of Human Erythrocytes in Vitro: Protective Effect of Albumin. International Journal of Molecular Sciences. 2015; 16(1):1728-1735. https://doi.org/10.3390/ijms16011728
Chicago/Turabian StyleRojas-Mancilla, Edgardo, Alexis Oyarce, Viviana Verdugo, Zhiping Zheng, and Rodrigo Ramírez-Tagle. 2015. "The Cluster [Re6Se8I6]3− Induces Low Hemolysis of Human Erythrocytes in Vitro: Protective Effect of Albumin" International Journal of Molecular Sciences 16, no. 1: 1728-1735. https://doi.org/10.3390/ijms16011728