Simultaneous Immobilization of Soil Cd(II) and As(V) by Fe-Modified Biochar
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cd-As Contaminated Soil Preparation and Characterization
2.2. Biochar Preparation, Modification and Characterization
2.3. Biochar Amendment on Cd-As Contaminated Soil
2.4. Sequential Extraction of Cd and As from Soils
2.5. Data Analysis
3. Results and Discussion
3.1. Characterization of Biochar
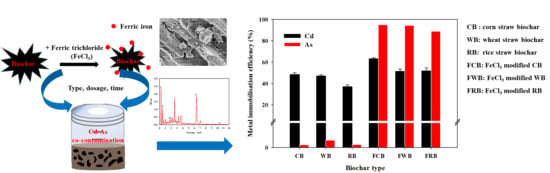
3.2. Effect of Biochar on Metal Immobilization
3.3. Metal Species and Soil Properties Respond to Biochar Amendments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, Y.M.; Tang, D.D.; Zhang, X.H.; Uchimiya, M.; Yuan, X.Y.; Li, M.; Chen, Y.Z. Effects of soil amendments on cadmium transfer along the lettuce-snail food chain: Influence of chemical speciation. Sci. Total Environ. 2019, 649, 801–807. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, P.; Welch, A.H.; Stollenwerk, K.G.; McLaughlin, M.J.; Bundschuh, J.; Panaullah, G. Arsenic in the environment: Biology and chemistry. Sci. Total Environ. 2007, 379, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Yin, D.; Wang, X.; Chen, C.; Peng, B.; Tan, C.; Li, H. Varying effect of biochar on Cd(II), Pb and As mobility in a multi-metal contaminated paddy soil. Chemosphere 2016, 152, 196–206. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Khan, S.; Ibrahim, M.; Sun, T.R.; Tang, J.-F.; Cotner, J.B.; Xu, Y.Y. Biochars induced modification of dissolved organic matter (DOM) in soil and its impact on mobility and bioaccumulation of arsenic and cadmium. J. Hazard. Mater. 2018, 348, 100–108. [Google Scholar] [CrossRef]
- Wang, Y.; Ji, H.; Lyu, H.; Liu, Y.; He, L.; You, L.; Zhou, C.; Yang, S. Simultaneous alleviation of Sb and Cd availability in contaminated soil and accumulation in Lolium multiflorum Lam. After amendment with Fe-Mn-Modified biochar. J. Clean. Prod. 2019, 231, 556–564. [Google Scholar] [CrossRef]
- Ali, S.; Rizwan, M.; Qayyum, M.F.; Ok, Y.S.; Ibrahim, M.; Riaz, M.; Arif, M.S.; Hafeez, F.; Al-Wabel, M.I.; Shahzad, A.N. Biochar soil amendment on alleviation of drought and salt stress in plants: A critical review. Environ. Sci. Pollut. Res. 2017, 24, 12700–12712. [Google Scholar] [CrossRef]
- O’Connor, D.; Peng, T.; Zhang, J.; Tsang, D.C.W.; Alessi, D.S.; Shen, Z.; Bolan, N.S.; Hou, D. Biochar application for the remediation of heavy metal polluted land: A review of in situ field trials. Sci. Total Environ. 2018, 619–620, 815–826. [Google Scholar] [CrossRef]
- Ahmad, M.; Rajapaksha, A.U.; Lim, J.E.; Zhang, M.; Bolan, N.; Mohan, D.; Vithanage, M.; Lee, S.S.; Ok, Y.S. Biochar as a sorbent for contaminant management in soil and water: A review. Chemosphere 2014, 99, 19–33. [Google Scholar] [CrossRef]
- Houben, D.; Evrard, L.; Sonnet, P. Mobility, bioavailability and pH-dependent leaching of cadmium, zinc and lead in a contaminated soil amended with biochar. Chemosphere 2013, 92, 1450–1457. [Google Scholar] [CrossRef]
- Yang, X.; Liu, J.; McGrouther, K.; Huang, H.; Lu, K.; Guo, X.; He, L.; Lin, X.; Che, L.; Ye, Z.; et al. Effect of biochar on the extractability of heavy metals (Cd, Cu, Pb, and Zn) and enzyme activity in soil. Environ. Sci. Pollut. Res. 2016, 23, 974–984. [Google Scholar] [CrossRef]
- Yang, X.; Lu, K.; McGrouther, K.; Che, L.; Hu, G.; Wang, Q.; Liu, X.; Shen, L.; Huang, H.; Ye, Z. Bioavailability of Cd and Zn in soils treated with biochars derived from tobacco stalk and dead pigs. J. Soils Sediments 2017, 17, 751–762. [Google Scholar] [CrossRef]
- Abbas, T.; Rizwan, M.; Ali, S.; Zia-Ur-Rehman, M.; Farooq Qayyum, M.; Abbas, F.; Hannan, F.; Rinklebe, J.; Sik Ok, Y. Effect of biochar on cadmium bioavailability and uptake in wheat (Triticum aestivum L.) grown in a soil with aged contamination. Ecotoxicol. Environ. Saf. 2017, 140, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Unzué-Belmonte, D.; Cornelis, J.T.; Linden, C.V.; Struyf, E.; Ronsse, F.; Delvaux, B. Effects of phytolithic rice-straw biochar, soil buffering capacity and pH on silicon bioavailability. Plant Soil 2019, 438, 187–203. [Google Scholar] [CrossRef]
- Li, Z.; Delvaux, B. Phytolith-rich biochar: A potential Si fertilizer in desilicated soils. GCB Bioenergy. 2019, 11, 1264–1282. [Google Scholar] [CrossRef] [Green Version]
- Maroušek, J.; Strunecký, O.; Stehel, V. Biochar farming: Defining economically perspective applications. Clean. Techn. Environ. Policy. 2019, 21, 1389–1395. [Google Scholar] [CrossRef]
- Vithanage, M.; Herath, I.; Joseph, S.; Bundschuh, J.; Bolan, N.; Ok, Y.S.; Kirkham, M.; Rinklebe, J. Interaction of arsenic with biochar in soil and water: A critical review. Carbon 2017, 113, 219–230. [Google Scholar] [CrossRef]
- Zhang, M.; Gao, B.; Varnoosfaderani, S.; Hebard, A.; Yao, Y.; Inyang, M. Preparation and characterization of a novel magnetic biochar for arsenic removal. Bioresour. Technol. 2013, 130, 457–462. [Google Scholar] [CrossRef]
- Rajapaksha, A.U.; Chen, S.S.; Tsang, D.C.; Zhang, M.; Vithanage, M.; Mandal, S.; Gao, B.; Bolan, N.S.; Ok, Y.S. Engineered/designer biochar for contaminant removal/immobilization from soil and water: Potential and implication of biochar modification. Chemosphere 2016, 148, 276–291. [Google Scholar] [CrossRef]
- Wang, S.; Gao, B.; Zimmerman, A.R.; Li, Y.; Ma, L.; Harris, W.G.; Migliaccio, K.W. Removal of arsenic by magnetic biochar prepared from pinewood and natural hematite. Bioresour. Technol. 2015, 175, 391–395. [Google Scholar] [CrossRef]
- Samsuri, A.W.; Sadegh-Zadeh, F.; Seh-Bardan, B.J. Adsorption of As (III) and As (V) by Fe coated biochars and biochars produced from empty fruit bunch and rice husk. J. Environ. Chem. Eng. 2013, 1, 981–988. [Google Scholar] [CrossRef]
- Li, H.; Dong, X.; da Silva, E.B.; de Oliveira, L.M.; Chen, Y.; Ma, L.Q. Mechanisms of metal sorption by biochars: biochar characteristics and modifications. Chemosphere 2017, 178, 466–478. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Kan, A.T.; Chen, W.; Tomson, M.B. pH-dependent effect of zinc on arsenic adsorption to magnetite nanoparticles. Water Res. 2010, 44, 5693–5701. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Huang, D.; Liu, X.; Meng, J.; Tang, C.; Xu, J. Remediation of As (III) and Cd(II) co-contamination and its mechanism in aqueous systems by a novel calcium-based magnetic biochar. J. Hazard. Mater. 2018, 348, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Fan, J.; Fu, M.; Ok, Y.S.; Hou, Y.; Cai, C. Adsorption antagonism and synergy of arsenate (V) and cadmium (II) onto Fe-modified rice straw biochars. Environ. Geochem. Health 2019, 41, 1755–1766. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhang, F.S.; Li, H.F.; Jiang, R.F. Accumulation of cadmium in the edible parts of six vegetable species grown in Cd-contaminated soils. J. Environ. Manage. 2009, 90, 1117–1122. [Google Scholar] [CrossRef]
- Cao, Q.; Hu, Q.H.; Baisch, C.; Khan, S.; Zhu, Y.G. Arsenate toxicity for wheat and lettuce in six Chinese soils with different properties. Environ. Toxicol. Chem. 2009, 28, 1946–1950. [Google Scholar] [CrossRef]
- Zhao, Q.; Zeng, D.H.; Fan, Z.P. Nitrogen and phosphorus transformations in the rhizospheres of three tree species in a nutrient-poor sandy soil. Appl. Soil. Ecol. 2010, 46, 341–346. [Google Scholar] [CrossRef]
- Wang, M.; Hu, R.; Zhao, J.; Kuzyakov, Y.; Liu, S. Iron oxidation affects nitrous oxide emissions via donating electrons to denitrification in paddy soils. Geoderma 2016, 271, 173–180. [Google Scholar] [CrossRef]
- Scheiner, D. Determination of ammonia and Kjeldahl nitrogen by indophenol method. Water Res. 1976, 10, 31–36. [Google Scholar] [CrossRef]
- Moorcroft, M.J.; Davis, J.; Compton, R.G. Detection and determination of nitrate and nitrite: A review. Talanta 2001, 54, 785–803. [Google Scholar] [CrossRef]
- Olsen, S.R.; Cole, C.V.; Watanabe, F.S.; Dean, L.A. Estimation of Available Phosphorus in Soils by Extraction With Sodium Bicarbonate; Department of Agriculture: Washington, DC, USA, 1954.
- Taylor, M.D. Determination of soil phosphorus in soil using simple Kjeldahl digestion. Commun. Soil Sci. Plan. 2000, 31, 2665–2670. [Google Scholar] [CrossRef]
- Reuter, D.; Robinson, J.B. Plant Analysis: An Interpretation Manual; CSIRO publishing: Clayton, Australia, 1997. [Google Scholar]
- Taylor, G.J.; Crowder, A.A. Use of the DCB technique for extraction of hydrous iron oxides from roots of wetland plants. Am. J. Bot. 1983, 70, 1254–1257. [Google Scholar] [CrossRef]
- Feng, M.H.; Shan, X.Q.; Zhang, S.; Wen, B. A comparison of the rhizosphere-based method with DTPA, EDTA, CaCl2, and NaNO3 extraction methods for prediction of bioavailability of metals in soil to barley. Environ. Pollut. 2005, 137, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Chancho, M.J.; López-Sánchez, J.F.; Schmeisser, E.; Goessler, W.; Francesconi, K.A.; Rubio, R. Arsenic speciation in plants growing in arsenic-contaminated sites. Chemosphere 2008, 71, 1522–1530. [Google Scholar] [CrossRef]
- Li, M.; Wang, Y.; Liu, M.; Liu, Q.; Xie, Z.; Li, Z.; Uchimiya, M.; Chen, Y. Three-year field observation of biochar-mediated changes in soil organic carbon and microbial activity. J. Environ. Qual. 2019, 48, 717–726. [Google Scholar] [CrossRef] [Green Version]
- Su, H.; Fang, Z.; Tsang, P.E.; Zheng, L.; Cheng, W.; Fang, J.; Zhao, D. Remediation of hexavalent chromium contaminated soil by biochar-supported zero-valent iron nanoparticles. J. Hazard. Mater. 2016, 318, 533–540. [Google Scholar] [CrossRef]
- Yu, K.; Xu, J.; Jiang, X.; Liu, C.; McCall, W.; Lu, J. Stabilization of heavy metals in soil using two organo-bentonites. Chemosphere 2017, 184, 884–891. [Google Scholar] [CrossRef]
- Shiowatana, J.; McLaren, R.G.; Chanmekha, N.; Samphao, A. Fractionation of arsenic in soil by a continuous-flow sequential extraction method. J. Environ. Qual. 2001, 30, 1940–1949. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Y.M.; Liu, Y.R.; Hu, H.Q.; He, J.Z. Mercury in soils of three agricultural experimental stations with long-term fertilization in China. Chemosphere 2008, 72, 1274–1278. [Google Scholar] [CrossRef]
- Beesley, L.; Marmiroli, M. The immobilisation and retention of soluble arsenic, cadmium and zinc by biochar. Environ. Pollut. 2011, 159, 474–480. [Google Scholar] [CrossRef]
- Micháleková-Richveisová, B.; Frišták, V.; Pipíška, M.; Ďuriška, L.; Moreno-Jimenez, E.; Soja, G. Iron-impregnated biochars as effective phosphate sorption materials. Environ. Sci. Pollut. Res. 2017, 24, 463–475. [Google Scholar] [CrossRef]
- Uchimiya, M.; Bannon, D.I.; Wartelle, L.H.; Lima, I.M.; Klasson, K.T. Lead retention by broiler litter biochars in small arms range soil: Impact of pyrolysis temperature. J. Agric. Food Chem. 2012, 60, 5035–5044. [Google Scholar] [CrossRef]
- Lu, K.; Yang, X.; Gielen, G.; Bolan, N.; Ok, Y.S.; Niazi, N.K.; Xu, S.; Yuan, G.; Chen, X.; Zhang, X. Effect of bamboo and rice straw biochars on the mobility and redistribution of heavy metals (Cd(II), Cu, Pb and Zn) in contaminated soil. J. Environ. Manag. 2017, 186, 285–292. [Google Scholar] [CrossRef]
- Xue, Q.; Ran, Y.; Tan, Y.; Peacock, C.L.; Du, H. Arsenite and arsenate binding to ferrihydrite organo-mineral coprecipitate: Implications for arsenic mobility and fate in natural environments. Chemosphere 2019, 224, 103–110. [Google Scholar] [CrossRef]
- Huff, M.D.; Lee, J.W. Biochar-surface oxygenation with hydrogen peroxide. J. Environ. Manag. 2016, 165, 17–21. [Google Scholar] [CrossRef]
- Lee, J.W.; Kidder, M.; Evans, B.R.; Paik, S.; Buchanan Iii, A.; Garten, C.T.; Brown, R.C. Characterization of biochars produced from cornstovers for soil amendment. Environ. Sci. Technol. 2010, 44, 7970–7974. [Google Scholar] [CrossRef] [Green Version]
- Gomez-Eyles, J.L.; Beesley, L.; Moreno-Jimenez, E.; Ghosh, U.; Sizmur, T. The potential of biochar amendments to remediate contaminated soils. Biochar Soil Biota 2013, 4, 100–133. [Google Scholar]
- Nagodavithane, C.L.; Singh, B.; Fang, Y. Effect of ageing on surface charge characteristics and adsorption behaviour of cadmium and arsenate in two contrasting soils amended with biochar. Soil Res. 2014, 52, 155–163. [Google Scholar] [CrossRef]
- Liang, J.; Xu, R.; Jiang, X.; Wang, Y.; Zhao, A.; Tan, W. Effect of arsenate on adsorption of Cd(II) by two variable charge soils. Chemosphere 2007, 67, 1949–1955. [Google Scholar] [CrossRef]
- Qian, L.; Chen, B. Dual role of biochars as adsorbents for aluminum: the effects of oxygen-containing organic components and the scattering of silicate particles. Environ. Sci. Technol. 2013, 47, 8759–8768. [Google Scholar] [CrossRef]
- Meng, J.; Tao, M.; Wang, L.; Liu, X.; Xu, J. Changes in heavy metal bioavailability and speciation from a Pb-Zn mining soil amended with biochars from co-pyrolysis of rice straw and swine manure. Sci. Total Environ. 2018, 633, 300–307. [Google Scholar] [CrossRef]
- Wan, X.; Dong, H.; Feng, L.; Lin, Z.; Luo, Q. Comparison of three sequential extraction procedures for arsenic fractionation in highly polluted sites. Chemosphere 2017, 178, 402–410. [Google Scholar] [CrossRef]
- Novotná, M.; Mikeš, O.; Komprdová, K. Development and comparison of regression models for the uptake of metals into various field crops. Environ Pollut. 2015, 207, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Ma, L.; Gao, B.; Harris, W. Dairy-manure derived biochar effectively sorbs lead and atrazine. Environ. Sci. Technol. 2009, 43, 3285–3291. [Google Scholar] [CrossRef] [PubMed]
- Qi, F.; Lamb, D.; Naidu, R.; Bolan, N.S.; Yan, Y.; Ok, Y.S.; Rahman, M.M.; Choppala, G. Cadmium solubility and bioavailability in soils amended with acidic and neutral biochar. Sci. Total Environ. 2018, 610, 1457–1466. [Google Scholar] [CrossRef] [PubMed]
- Schonfield, R.K. Can a precise meaning be given to ‘available’ soil phosphorus? Soils Fertilizers 1955, 18, 373–375. [Google Scholar]
- Maroušek, J.; Stehel, V.; Vochozka, M.; Kolář, L.; Maroušková, A.; Strunecký, O.; Peterka, J.; Kopecký, M.; Shreedhar, S. Ferrous sludge from water clarification: Changes in waste management practices advisable. J. Clean. Prod. 2019, 218, 459–464. [Google Scholar] [CrossRef]
- Rout, G.R.; Sahoo, S. Role of iron in plant growth and metabolism. Rev. Agric. Sci. 2015, 3, 1–24. [Google Scholar] [CrossRef] [Green Version]




| Treatment | pH | Inorganic N | Available P | DTPA-Fe |
|---|---|---|---|---|
| mg/kg | ||||
| Control | 4.86 ± 0.001a | 112 ± 0.91g | 9.19 ± 0.11d | 116 ± 1.81c |
| CB | 4.89 ± 0.01a | 90.2 ± 1.38f | 8.18 ± 0.05c | 81.6 ± 2.64b |
| WB | 4.82 ± 0.08a | 82.4 ± 0.16e | 7.11 ± 0.47b | 71.7 ± 1.53a |
| RB | 4.85 ± 0.04a | 80.0 ± 0.21d | 6.44 ± 0.47a | 82.3 ± 4.21b |
| FCB | 5.25 ± 0.02c | 67.5 ± 0.04c | 9.25 ± 0.35d | 195 ± 4.11f |
| FWB | 5.20 ± 0.01b | 58.9 ± 0.37b | 6.77 ± 0.47a | 181 ± 3.29e |
| FRB | 5.21 ± 0.04bc | 48.0 ± 0.45a | 7.02 ± 0.59b | 176 ± 2.69d |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.-m.; Wang, S.-w.; Wang, C.-q.; Zhang, Z.-y.; Zhang, J.-q.; Meng, M.; Li, M.; Uchimiya, M.; Yuan, X.-y. Simultaneous Immobilization of Soil Cd(II) and As(V) by Fe-Modified Biochar. Int. J. Environ. Res. Public Health 2020, 17, 827. https://doi.org/10.3390/ijerph17030827
Wang Y-m, Wang S-w, Wang C-q, Zhang Z-y, Zhang J-q, Meng M, Li M, Uchimiya M, Yuan X-y. Simultaneous Immobilization of Soil Cd(II) and As(V) by Fe-Modified Biochar. International Journal of Environmental Research and Public Health. 2020; 17(3):827. https://doi.org/10.3390/ijerph17030827
Chicago/Turabian StyleWang, Yi-min, Shao-wei Wang, Cheng-qian Wang, Zhi-yuan Zhang, Jia-qi Zhang, Meng Meng, Ming Li, Minori Uchimiya, and Xu-yin Yuan. 2020. "Simultaneous Immobilization of Soil Cd(II) and As(V) by Fe-Modified Biochar" International Journal of Environmental Research and Public Health 17, no. 3: 827. https://doi.org/10.3390/ijerph17030827