Effect of the Surface Chemical Composition and of Added Metal Cation Concentration on the Stability of Metal Nanoparticles Synthesized by Pulsed Laser Ablation in Water
Abstract
:1. Introduction
2. Materials and Methods
2.1. NP Synthesis and Preliminary Characterization
2.2. Electron Microscopy
2.3. XPS
3. Results and Discussion
3.1. Size and Surface Chemical Composition
3.2. Particle ζ-Potential
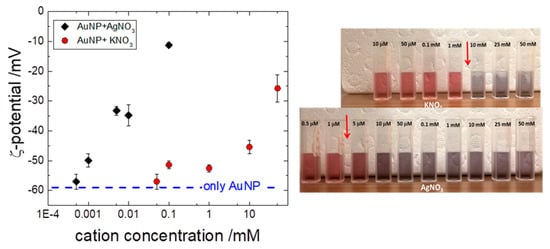
3.3. Effect of Added Metal Cations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ramalingam, V. Multifunctionality of gold nanoparticles: Plausible and convincing properties. Adv. Colloid Interface Sci. 2019, 271, 101989. [Google Scholar] [CrossRef]
- Ou, J.; Zhou, Z.; Chen, Z.; Tan, H. Optical Diagnostic Based on Functionalized Gold Nanoparticles. Int. J. Mol. Sci. 2019, 20, 4346. [Google Scholar] [CrossRef] [Green Version]
- Gellé, A.; Moores, A. Plasmonic nanoparticles: Photocatalysts with a bright future. Curr. Opin. Green Sustain. Chem. 2019, 15, 60–66. [Google Scholar] [CrossRef]
- Maiolo, D.; Paolini, L.; Di Noto, G.; Zendrini, A.; Berti, D.; Bergese, P.; Ricotta, D. Colorimetric nanoplasmonic assay to determine purity and titrate extracellular vesicles. Anal. Chem. 2015, 87, 4168–4176. [Google Scholar] [CrossRef]
- Monopoli, A.; Cotugno, P.; Palazzo, G.; Ditaranto, N.; Mariano, B.; Cioffi, N.; Ciminale, F.; Nacci, A. Ullmann Homocoupling Catalysed by Gold Nanoparticles in Water and Ionic Liquid. Adv. Synth. Catal. 2012, 354, 2777–2788. [Google Scholar] [CrossRef]
- Dell’Aglio, M.; Salajková, Z.; Mallardi, A.; Mezzenga, R.; van’t Hag, L.; Cioffi, N.; Palazzo, G.; De Giacomo, A. Application of gold nanoparticles embedded in the amyloids fibrils as enhancers in the laser induced breakdown spectroscopy for the metal quantification in microdroplets. Spectrochim. Acta Part B At. Spectrosc. 2019, 155, 115–122. [Google Scholar] [CrossRef]
- Dell’Aglio, M.; Alrifai, R.; De Giacomo, A. Nanoparticle Enhanced Laser Induced Breakdown Spectroscopy (NELIBS), a first review. Spectrochim. Acta Part B At. Spectrosc. 2018, 148, 105–112. [Google Scholar] [CrossRef]
- Kudera, S.; Manna, L. Nanostructured Fluids. In Colloidal Foundations of Nanoscience; Berti, D., Palazzo, G., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 47–80. [Google Scholar]
- Hamad-Schifferly, K. Stability of Dispersions and Interactions. In Colloidal Foundations of Nanoscience; Berti, D., Palazzo, G., Eds.; Newnes: Amsterdam, The Netherlands, 2014; pp. 33–46. [Google Scholar]
- Barcikowski, S.; Compagnini, G. Advanced nanoparticle generation and excitation by lasers in liquids. Phys. Chem. Chem. Phys. 2013, 15, 3022–3026. [Google Scholar] [CrossRef] [PubMed]
- Dell’Aglio, M.; Gaudiuso, R.; De Pascale, O.; De Giacomo, A. Mechanisms and processes of pulsed laser ablation in liquids during nanoparticle production. Appl. Surf. Sci. 2015, 348, 4–9. [Google Scholar] [CrossRef]
- Sylvestre, J.P.; Poulin, S.; Kabashin, A.V.; Sacher, E.; Meunier, M.; Luong, J.H. Surface Chemistry of Gold Nanoparticles Produced by Laser Ablation in Aqueous Media. J. Phys. Chem. B 2004, 108, 16864–16869. [Google Scholar] [CrossRef]
- De Anda Villa, M.; Gaudin, J.; Amans, D.; Boudjada, F.; Bozek, J.; Evaristo Grisenti, R.; Lamour, E.; Laurens, G.; Macé, S.; Nicolas, C.; et al. Assessing the Surface Oxidation State of Free-Standing Gold Nanoparticles Produced by Laser Ablation. Langmuir 2019, 35, 11859–11871. [Google Scholar] [CrossRef] [PubMed]
- Muto, H.; Yamada, K.; Miyajima, K.; Mafuné, F. Estimation of surface oxide on surfactant-free gold nanoparticles laser-ablated in water. J. Phys. Chem. C 2007, 111, 17221–17226. [Google Scholar] [CrossRef]
- Merk, V.; Rehbock, C.; Becker, F.; Hagemann, U.; Nienhaus, H.; Barcikowski, S. In situ non-DLVO stabilization of surfactant-free, plasmonic gold nanoparticles: Effect of Hofmeister’s anions. Langmuir 2014, 30, 4213–4222. [Google Scholar] [CrossRef]
- Palazzo, G.; Valenza, G.; Dell’Aglio, M.; De Giacomo, A. On the stability of gold nanoparticles synthesized by laser ablation in liquids. J. Colloid Interface Sci. 2016, 489, 47–56. [Google Scholar] [CrossRef]
- Dell’Aglio, M.; Motto-Ros, V.; Pelascini, F.; Gornushkin, I.B.; Giacomo, A. De Investigation on the material in the plasma phase by high temporally and spectrally resolved emission imaging during pulsed laser ablation in liquid (PLAL) for NPs production and consequent considerations on NPs formation. Plasma Sources Sci. Technol. 2019, 28, 085017. [Google Scholar] [CrossRef]
- Dell’Aglio, M.; De Giacomo, A. Plasma charging effect on the nanoparticles releasing from the cavitation bubble to the solution during nanosecond Pulsed Laser Ablation in Liquid. Appl. Surf. Sci. 2020, 515, 146031. [Google Scholar] [CrossRef]
- Taccogna, F.; Dell’Aglio, M.; Rutigliano, M.; Valenza, G.; Giacomo, A. De On the growth mechanism of nanoparticles in plasma during pulsed laser ablation in liquids. Plasma Sources Sci. Technol. 2017, 26, 045002. [Google Scholar] [CrossRef]
- Dell’Aglio, M.; Mangini, V.; Valenza, G.; De Pascale, O.; De Stradis, A.; Natile, G.; Arnesano, F.; De Giacomo, A. Silver and gold nanoparticles produced by pulsed laser ablation in liquid to investigate their interaction with Ubiquitin. Appl. Surf. Sci. 2016, 374, 297–304. [Google Scholar] [CrossRef]
- Mateos, H.; Valentini, A.; Colafemmina, G.; Murgia, S.; Robles, E.; Brooker, A.; Palazzo, G. Binding isotherms of surfactants used in detergent formulations to bovine serum albumin. Colloids Surf. A Physicochem. Eng. Asp. 2020, 598. [Google Scholar] [CrossRef]
- Mateos, H.; Valentini, A.; Robles, E.; Brooker, A.; Cioffi, N.; Palazzo, G. Measurement of the zeta-potential of solid surfaces through Laser Doppler Electrophoresis of colloid tracer in a dip-cell: Survey of the effect of ionic strength, pH, tracer chemical nature and size. Colloids Surf. A Physicochem. Eng. Asp. 2019, 576, 82–90. [Google Scholar] [CrossRef]
- Casiello, M.; Picca, R.; Fusco, C.; D’Accolti, L.; Leonardi, A.; Lo Faro, M.; Irrera, A.; Trusso, S.; Cotugno, P.; Sportelli, M.; et al. Catalytic Activity of Silicon Nanowires Decorated with Gold and Copper Nanoparticles Deposited by Pulsed Laser Ablation. Nanomaterials 2018, 8, 78. [Google Scholar] [CrossRef] [Green Version]
- Radnik, J.; Mohr, C.; Claus, P. On the origin of binding energy shifts of core levels of supported gold nanoparticles and dependence of pretreatment and material synthesis. Phys. Chem. Chem. Phys. 2003, 5, 172–177. [Google Scholar] [CrossRef]
- Naumkin, A.V.; Kraut-Vass, A.; Gaarenstroom, S.W.; Powell, C.J. NIST X-ray Photoelectron Spectroscopy Database. Meas. Serv. Div. Natl. Inst. Stand. Technol. 2012, 20899, 20899. [Google Scholar] [CrossRef]
- Fischer, M.; Hormes, J.; Marzun, G.; Wagener, P.; Hagemann, U.; Barcikowski, S. In Situ Investigations of Laser-Generated Ligand-Free Platinum Nanoparticles by X-ray Absorption Spectroscopy: How Does the Immediate Environment Influence the Particle Surface? Langmuir 2016, 32, 8793–8802. [Google Scholar] [CrossRef]
- Hunter, R.J. Zeta Potential in Colloidal Science; Academic press: Cambridge, MA, USA, 2013. [Google Scholar]
- Aldewachi, H.; Chalati, T.; Woodroofe, M.N.; Bricklebank, N.; Sharrack, B.; Gardiner, P. Gold nanoparticle-based colorimetric biosensors. Nanoscale 2018, 10, 18–33. [Google Scholar] [CrossRef] [Green Version]
- Israelachvili, J.N. Intermolecular and Surface Forces; Elsevier: Amsterdam, The Netherlands, 2011; ISBN 9780123751829. [Google Scholar]





| Size (Averaged TEM Diameter) | NPs Concentration | ||
|---|---|---|---|
| nm | nmol·NPs/L·(nM) | n°·NPs/L | |
| AuNPs | 11 ± 5 | 8.8 | 5.3 × 1015 |
| AgNPs | 11 ± 5 | 3.5 | 2.2 × 1015 |
| PtNPs | 11 ± 5 | 6.6 | 4.0 × 1015 |
| Size (Diameter)/nm | ||||||
|---|---|---|---|---|---|---|
| SPR | DLS | TEM | ζ-Potential | XPS Oxidation | ||
| Xc (nm) | Average | (mV) | ||||
| AuNPs | 9 ± 2 | 10.2 ± 0.6 | 10.2 ± 0.2 | 11 ± 5 | −62 ± 7 | Au(0) 97.1% |
| Au+ 2.9% | ||||||
| AgNPs | 8 ± 2 | 13 ± 2 | 10.6 ± 0.4 | 11 ± 5 | −44 ± 2 | Ag(0) 100% |
| PtNPs | - | 14 ± 2 | 11.3 ± 0.3 | 11 ± 5 | −58 ± 3 | Pt(0) 77.6% |
| Pt(II) 10.8% | ||||||
| Pt(IV) 11.6% | ||||||
| Element% | AuNPs | AgNPs | PtNPs |
|---|---|---|---|
| C% | 22.2 | 30.8 | 31.9 |
| O% | 28.2 | 28.3 | 29.3 |
| Si% | 33.0 | 25.5 | 20.4 |
| K% | 2.4 | 2.2 | 4.5 |
| Cl% | 2.8 | 1.6 | 4.3 |
| Au% | 11.4 | / | / |
| Ag% | / | 11.6 | / |
| Pt% | / | / | 9.6 |
| Ion | E°/V | ccc/μM | ccc/μM from Equation (3) |
|---|---|---|---|
| Ag+ | 0.8 | 3 ± 2 | 50 × 103 |
| Cu++ | 0.34 | 6 ± 2 | 800 |
| Pb++ | −0.13 | 13 ± 5 | 800 |
| Mg++ | −2.36 | 150 ± 50 | 800 |
| Na+ | −2.71 | (35 ± 10) × 103 | 50 × 103 |
| Ca++ | −2.87 | 150 ± 50 | 800 |
| K+ | −2.93 | (8 ± 2) × 103 | 50 × 103 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mateos, H.; Picca, R.A.; Mallardi, A.; Dell’Aglio, M.; De Giacomo, A.; Cioffi, N.; Palazzo, G. Effect of the Surface Chemical Composition and of Added Metal Cation Concentration on the Stability of Metal Nanoparticles Synthesized by Pulsed Laser Ablation in Water. Appl. Sci. 2020, 10, 4169. https://doi.org/10.3390/app10124169
Mateos H, Picca RA, Mallardi A, Dell’Aglio M, De Giacomo A, Cioffi N, Palazzo G. Effect of the Surface Chemical Composition and of Added Metal Cation Concentration on the Stability of Metal Nanoparticles Synthesized by Pulsed Laser Ablation in Water. Applied Sciences. 2020; 10(12):4169. https://doi.org/10.3390/app10124169
Chicago/Turabian StyleMateos, Helena, Rosaria A. Picca, Antonia Mallardi, Marcella Dell’Aglio, Alessandro De Giacomo, Nicola Cioffi, and Gerardo Palazzo. 2020. "Effect of the Surface Chemical Composition and of Added Metal Cation Concentration on the Stability of Metal Nanoparticles Synthesized by Pulsed Laser Ablation in Water" Applied Sciences 10, no. 12: 4169. https://doi.org/10.3390/app10124169